Biomedical Engineering Reference
In-Depth Information
different, tissue types. One method for aligning
imagery to the patient is to use some form of extrinsic
marker
and scanning bar has three degrees of freedom to allow
easy placement of the bar in desired configurations.
a set of landmarks or other frame structures
that attach to the patient prior to imaging, and that
can then be used to establish a correspondence be-
tween position in the image and position on the patient.
Examples include stereotactic frames, bone screws, and
skin markers (e.g.,
[3, 11, 15, 16, 18, 19, 23-26, 28]
).
In other words, by placing markers on the patient
prior to imaging, and keeping them rigidly attached
through the completion of surgery, one obtains a co-
ordinate frame visible in the imagery that directly sup-
ports transference of information to the surgical field
of view. Stereotactic frames, though, not only are un-
comfortable for the patient, but are cumbersome for the
surgeon. They are limited to guidance along fixed paths
and prevent access to some parts of the head. We would
like to use a frameless system both for its simplicity and
generality, and for its potential for use in other parts of
the body. More recently, frameless stereotaxy systems
have been pursued by many groups (e.g.,
[1, 2, 6, 21, 29,
35]
) and usually consist of two components: registration
and tracking. We have added a third, initial, component
to our system
d
6.5.2.1 Imagery subsystem
MRI is the prime imaging modality for the neurosurgery
cases we support. The images are acquired prior to sur-
gery with no need for special landmarking strategies. To
use the imagery, it is important to create detailed models
of the tissues being imaged. This means that we must
segment the images: identify the type of tissue associated
with each voxel (or volume element) in the imagery, and
then create connected geometric models of the different
types of tissue. A wide range of methods (e.g.,
[27, 30,
33, 34, 37]
) have been applied to the segmentation
problem. Classes of methods include statistical classifiers
(e.g.,
[33, 37]
), which use variations in recorded tissue
response to label individual elements of the medical scan,
then extract surface boundaries of connected tissue re-
gions to create structural models; deformable surface
methods (e.g.,
[27, 30]
), which directly fit boundary
models to delineations between adjacent tissue types;
and atlas-driven segmenters (e.g.,
[34]
), which use ge-
neric models of standard anatomy to guide the labeling
and segmentation of new scans.
Our current approach to segmentation uses an auto-
mated method to initially segment into major tissue
classes while removing gain artifacts from the imagery
[17,37]
, then uses operator-driven interactive tools to
refine this segmentation. This latter step primarily relies
on 3D visualization and data manipulation techniques to
correct and refine the initial automated segmentation.
The segmented tissue types include skin, used for reg-
istration, and internal structures such as white matter,
gray matter, tumor, vessels, cerebrospinal fluid, and
structures. These segmented structures are processed by
the Marching Cube algorithm
[20]
to construct isosur-
faces and to support surface rendering for visualization.
The structural models of patients constructed using
such methods can be augmented with functional in-
formation. For example, functional MRI methods or
transcranial magnetic stimulation methods (e.g.,
[9]
) can
be used to identify motor or sensory cortex. The key
issue is then merging this data with the structural
models, and to do this we use a particular type of regis-
tration method
[7,8,38]
. This approach uses stochastic
sampling to find the registration that optimizes the
mutual information between the two data sets. Opti-
mizing mutual information makes the method insensitive
to intensity differences between the two sensory mo-
dalities, and hence it can find the best alignment even
if different anatomical features are highlighted in the
scans.
reconstructed models of the patient's
anatomy. The system's components are described next,
with emphasis on the use of registration to align imagery
with patient and surgeon's viewpoint.
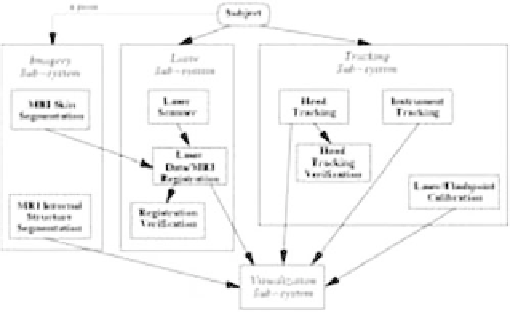
The architecture of our image-guided surgery system
(
Fig. 6.5-1
) supports frameless, nonfiducial, registration
of medical imagery by matching surface data between
patient and image model. The system consists of a por-
table cart (
Fig. 6.5-2
) containing a Sun UltraSPARC
workstation and the hardware to drive the laser scanner
and Flashpoint tracking system. On top of the cart
is mounted an articulated extendable arm to which we
attach a bar housing the laser scanner and Flashpoint
cameras. The three linear Flashpoint cameras are inside
the bar. The laser is attached to one end of the bar, and
a video camera to the other. The joint between the arm
d
Figure 6.5-1 Image-guided surgery system architecture.