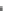Biomedical Engineering Reference
In-Depth Information
0.8
Fibrinogen on silastic rubber
Albumin on silastic rubber
Fibrinogen on PyC
Albumin on Pyc
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
0
10
20
30
40
50
60
70
80
90
100
110
Time (min)
Fig. 3.2.11-11 Fibrinogen and albumin adsorption on pyrolytic carbon (PyC) and Silastic SR.
observed a significant difference in platelet reaction with
pyrolytic carbon beads in packed columns prior to and
after pretreatment with albumin. With no albumin pre-
conditioning treatment, platelet retention by the columns
was high, but the release of platelet constituents was low.
However, with albumin pretreatment, platelet retention
and the release of constituents was minimal.
The foregoing observations led to the view that py-
rolytic carbon owes its demonstrated blood compatibility
to its inertness and to its ability to quickly absorb pro-
teins from blood without triggering a protein-denaturing
reaction (Haubold
et al.
, 1981; Nyilas and Chiu, 1978).
However, the assertion that pyrolytic carbon is an inert
material and induces minimal conformational changes in
adsorbed protein was reexamined by Feng and Andrade
(1994). Using DSC and a variety of proteins and buffers,
they found that pyrolytic carbon surfaces denatured all of
the proteins studied. They concluded that whether or
not a surface denatures protein cannot be the sole criteria
for blood compatibility. Their suggestion was that the
specific proteins and the sequence in which they are
denatured may be important. For example, it was
suggested that pyrolytic carbon may first adsorb and
denature albumin, which forms a layer that subsequently
passivates the surface and inhibits thrombosis.
Chinn
et al.
(1994) reexamined the adsorption of al-
bumin and fibrinogen on pyrolytic carbon surfaces and
noted that relatively large amounts of fibrinogen were
adsorbed and speculated that the adsorbed fibrinogen
was rapidly converted to a non-elutable form. If the
elutable form is more reactive to platelets than the
nonelutable form, then the nonelutable protein layer may
contribute to the passivating effect.
Work on visualizing the carbon surface and platelet
adhesion done by Goodman
et al.
(1995) using
low-accelerating-voltage SEM, along with critical-point
drying techniques, has discovered that the platelet
spreading on pyrolytic carbon surfaces is more extensive
than previously observed (Haubold
et al.
, 1981). How-
ever, platelet loading was in a static flow situation that
does not model the physiological flow that a heart valve is
subjected to. Hence, this approach cannot resolve kinetic
effects on platelet adhesion. However, Okazaki, Tweden,
and co-workers observed adherent platelets on valves
following implantation in sheep that were not treated
26
24
22
Fibrinogen on Glass 37
C
Fibrinogen on Glass 25
°
C
Fibrinogen on PyC 37
20
°
C
Fibrinogen on PyC 25
°
C
°
18
16
14
12
10
8
6
4
2
0
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
1.8
2.0
2.2
2.4
Relative surface coverage
Fig. 3.2.11-12 Integral heat of sorption for fibrinogen on glass
and fibrinogen on PyC at two different temperatures (Nyilas and
Chiu, 1978).