Biomedical Engineering Reference
In-Depth Information
The route and conditions under which synthetic HA is
produced will greatly influence its physical and chemical
characteristics. Factors that affect the rate of resorption
of the implant include physical factors such as the
physical features of the material (e.g., surface area,
crystallite size), chemical factors such as atomic and ionic
substitutions in the lattice, and biological factors such as
the types of cells surrounding the implant and location,
age, species, sex, and hormone levels.
The thermodynamic stability of the various calcium
phosphates is summarized in the form of the phase dia-
gram shown in
Fig. 3.2.10-8
. The binary equilibrium
phase diagram between CaO and P
2
O
5
gives an indication
of the compounds formed between the two oxides, and
by comparing this with
Table 3.2.10-7
it is possible to
identify the naturally occurring calcium phosphate min-
erals. The diagram does not indicate the phase boundaries
of apatite due to the absence of hydroxyl groups.
However, from the binary diagram an indication may
be obtained of the stability of other calcium phosphates
with temperature.
The stoichiometry of HA is highly significant where
thermal processing of the material is required. Slight
imbalances in the stoichiometric ratio of calcium and
phosphorus in HA (from the standard molar ratio of
1.67), can lead to the appearance of either a or
b-TCP on heat treatment. Many early papers concerning
the production and processing of HA powders reported
problems in avoiding the formation of these extraneous
phases (Jarcho
et al.
, 1976; De With
et al.
, 1981a, b;
Peelen
et al.
, 1978). However, using stoichiometric HA
it should be possible to sinter, without phase purity
problems, at temperatures in excess of 1300
C.
20000
15000
1000
5000
0
10
20
30
2 Theta (degrees)
40
50
60
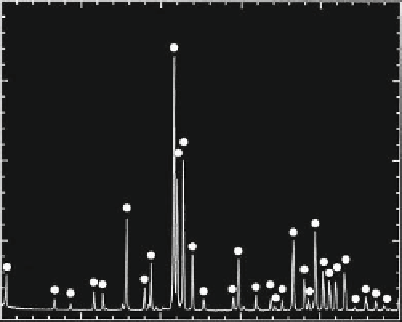
Fig. 3.2.10-9 X-ray diffraction of HA.
X-ray diffraction (
Fig. 3.2.10-9
) and infra-red spec-
troscopy (
Fig. 3.2.10-10
) should be used to reveal the
phase purity and level of hydroxylation of HA. Kijima
and Tsutsumi (1979) used these techniques to study HA
sintered at different temperatures and reported that
after sintering at 900
C, the material was fully hydrox-
ylated, but after sintering at temperatures higher than
this, dehydroxylation occurred. Dehydration of HA,
produced by processes such as high temperature solid
state reaction, result in the formation of oxyhydrox-
yapatite: Ca
10
(PO
4
)
6
(OH)
2
2x
O
x
V
x
(where V is a hy-
droxyl vacancy). HA has a
P6
3
/m
space group: This
signifies that the lattice is primitive Bravais, there is
a sixfold axis parallel to the
c
axis and
a
1/2 (3/6)
translation along the length of the
c
axis (a screw axis)
with a mirror plane situated perpendicular to the screw
axis and the
c
axis. The
a
and
c
parameters for HA are
0.9418 nm and 0.6884 nm, respectively.
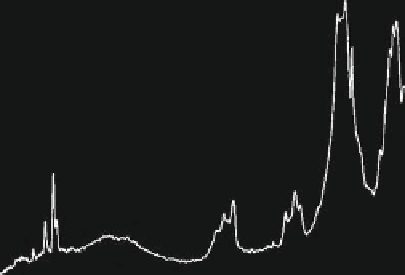
−
PO
3
ν
3
ν
4
EP-1
ν
1
−
PO
3
ν
2
−
(
ν
3
)
CO
2
OH
−
(Stretch)
A & B
−
(Absorbed)
CO
2
OH
−
(Libration)
A
B
A & B
H
2
O
(Absorbed)
−
(
ν
2
)
CO
2
Fig. 3.2.10-8 Phase equilibrium diagram of calcium phosphates
in a water atmosphere. Shaded area is processing range to
yield HA-containing implants. (After K. de Groot. 1988. Ann. N. Y.
Acad. Sci. 523: 227.)
4000 3600
3200
2800 2400 2000
Wavenumber cm
−
1
1600
1200
800
400
Fig. 3.2.10-10 Typical FT-IR spectrum for HA.