Biomedical Engineering Reference
In-Depth Information
and the binder is burned out, resulting in bisque ware. At
a very much higher temperature, the part is densified
during firing. After cooling to ambient temperature, one
or more finishing steps may be applied, such as polishing.
Porous ceramics are produced by adding a second phase
that decomposes prior to densification, leaving behind
holes or pores (Schors and Holmes, 1993), or trans-
forming natural porous organisms, such as coral, to
porous
Table 3.2.10-3 Bioceramic material characteristics and properties
Composition
Microstructure
Number of phases
Percentage of phases
Distribution of phases
HA
by
hydrothermal
processing
(Roy
and
Linnehan, 1974).
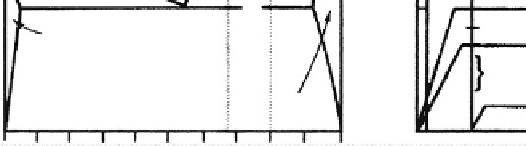
The interrelation between microstructure and
thermal processing of various bioceramics is shown in
Fig. 3.2.10-3
, which is a binary phase diagram consisting of
a network-forming oxide such as SiO
2
(silica), and some
arbitrary network modifier oxide (MO) such as CaO.
When a powdered mixture of MO and SiO
2
is heated to
the melting temperature
T
m
,
the entire mass will become
liquid (L). The liquid will become homogeneous when
held at this temperature for a sufficient length of time.
When the liquid is cast (paths 1B, 2, 5), forming the shape
of the object during the casting, either a glass or a poly-
crystalline microstructure will result. Plasma spray coat-
ing follows path 1A. However, a network-forming oxide is
not necessary to produce plasma-sprayed coatings such as
HAs, which are polycrystalline (Lacefield, 1993).
If the starting composition contains a sufficient
quantity of network former (SiO
2
), and the casting rate
is sufficiently slow, a glass will result (path 1B). The
viscosity of the melt increases greatly as it is cooled, until
at approximately
T
1
, the glass transition point, the ma-
terial is transformed into a solid.
If either of these conditions is not met, a polycrystalline
microstructure will result. The crystals begin growing at
T
L
and complete growth at T
2
. The final material consists
of the equilibrium crystalline phases predicted by the
Size of phases
Connectivity of phases
Phase state
Crystal structure
Defect structure
Amorphous structure
Pore structure
Surface
Flatness
Finish
Composition
Second phase
Porosity
Shape
particulates with water and an organic binder, then
pressing them in a mold. This is termed ''forming.'' The
formed piece is called green ware. Subsequently, the
temperature is raised to evaporate the water (i.e., drying)
A
B
T
M
x
x
T
Plasma spraying
M
Path (1A)
Melting &
homogenization
T
M
T
L
L
1
+L
2
L
T
L
T
S
L+SiO
2(ss)
x
T
L+MO
(ss)
3
Liquid phase
sintering
Solid-state
sintering
(3)
T
2
x T
4
x
x
T
2
(2)
MO
(ss)
MO
(ss)
+SiO
2(ss)
(4)
T
Glass
transformation
1
Ceraming
(1)
5b
SiO
2(ss)
5a
Log time
MO
10
30
50
70
90
SiO
2
Weight %
Fig. 3.2.10-3 Relation of thermal processing schedules of various bioceramics to equilibrium phase diagram.