Biomedical Engineering Reference
In-Depth Information
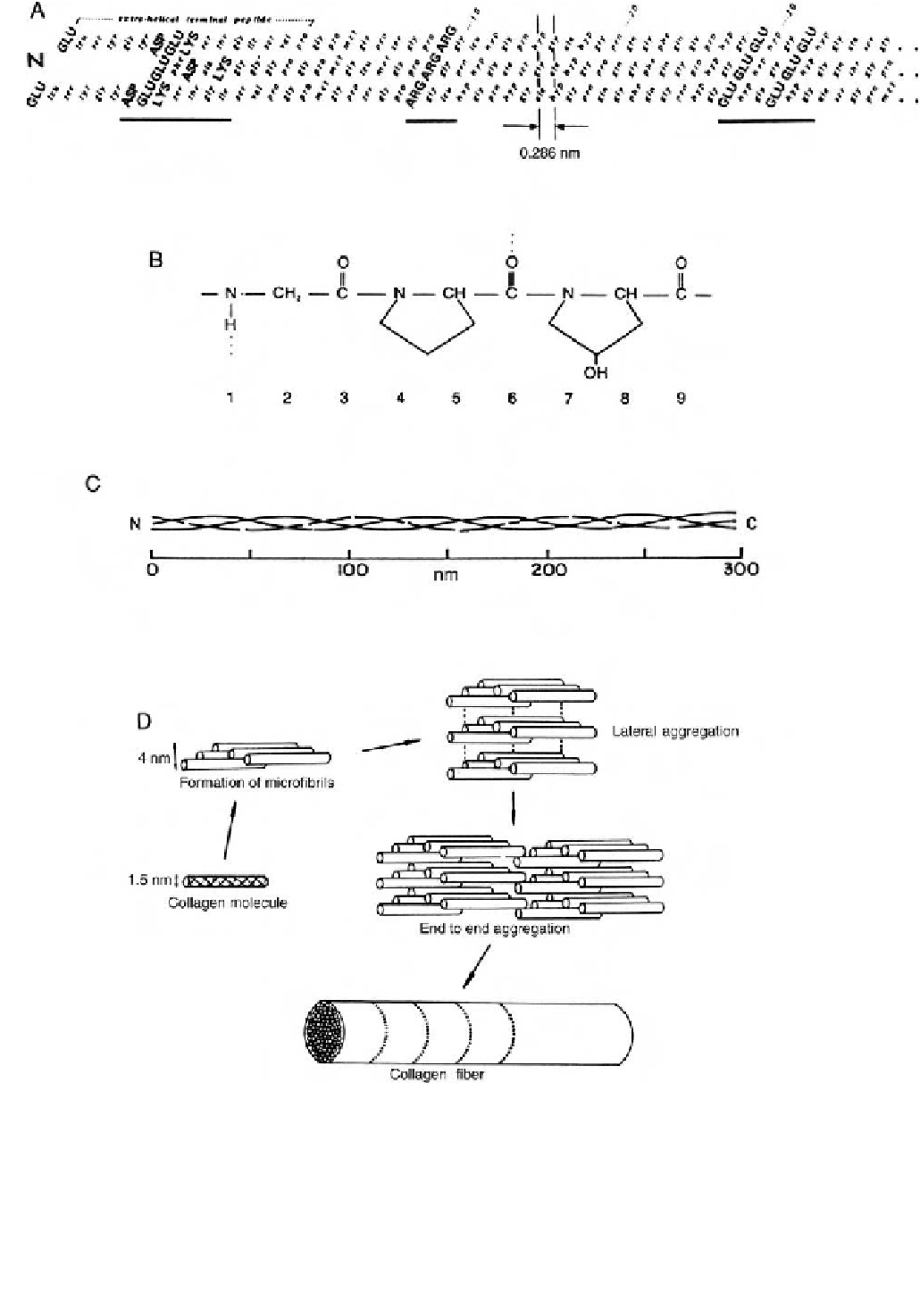
Fig. 3.2.8-1 Collagen, like other proteins, is distinguished by several levels of structural order. (A) Primary structure
d
the complete se-
quence of amino acids along each polypeptide chain. An example is the triple chain sequence of type I calf skin collagen at the N-end of
the molecule. Roughly 5% of a complete molecule is shown above. No attempt has been made to indicate the coiling of the chains. Amino
acid residues participating in the triple helix are numbered, and the residue-to-residue spacing (0.286 nm) is shown as a constant within
the triple helical domain, but not outside it. Bold capitals indicate charged residues which occur in groups (underlined). (Reprinted from
J. A. Chapman and D. J. S. Hulmes (1984). In Ultrastructure of the Connective Tissue Matrix, A. Ruggeri and P. M. Motta, eds. Martinus
Nijhoff, Boston, Chap. 1,
Fig. 3.2.8-1
, p. 2, with permission.) (B) Secondary structure
d
the local configuration of a polypeptide chain. The