Biomedical Engineering Reference
In-Depth Information
between the eroding surface layer and the intact polymer
in the core of the device (
Mathiowitz
et al.
, 1990
).
Surface eroding devices have so far been obtained only
from a small number of hydrophobic polymers
containing hydrolytically highly reactive linkages in the
backbone. A possible exception to this general rule is
enzymatic surface erosion. The inability of enzymes to
penetrate into the interior of a solid, polymeric device
may result in an enzyme-mediated surface erosion
mechanism. Currently, polyanhydrides and poly(ortho
esters) are the best known examples of polymers that can
be fabricated into surface eroding devices.
chemical degradation mechanisms have been identified
(
Fig. 3.2.7-2
)(
Rosen
et al.
, 1988
). Chemical reactions
can lead to cleavage of crosslinks between water-soluble
polymer chains (mechanism I), to the cleavage of poly-
mer side chains resulting in the formation of polar or
charged groups (mechanism II), or to the cleavage of the
polymer backbone (mechanism III). Obviously, combi-
nations of these mechanisms are possible: for instance,
a cross-linked polymer may first be partially solubilized
by the cleavage of crosslinks (mechanism I), followed by
the cleavage of the backbone itself (mechanism III). It
should be noted that water is key to all of these degra-
dation schemes. Even enzymatic degradation occurs in
aqueous environment.
Since the chemical cleavage reactions described above
can be mediated by water or by biological agents such as
enzymes and microorganisms, it is possible to distinguish
between hydrolytic degradation and biodegradation, re-
spectively. It has often been stated that the availability of
water is virtually constant in all soft tissues and varies little
Mechanisms of chemical degradation
Although bioerosion can be caused by the solubilization
of an intact polymer, chemical degradation of the poly-
mer is usually the underlying cause for the bioerosion of
a
solid,
polymeric
device.
Several
distinct
types
of
Water
insoluble
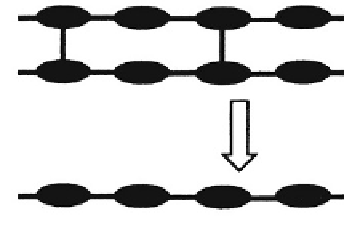
Mechanism I:
Cleavage of crosslinks
between water soluble
polymer chains
Water soluble
Water
insoluble
X
X
X
X
X
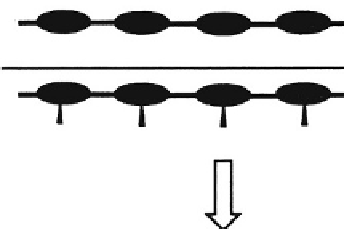
Mechanism II:
Transformation or cleavage of side chains (X)
leading to the formation of polar or charged
groups (Y)
Water soluble
Y
Y
Y
Y
Y
Water
insoluble
Mechanism III:
Cleavage of backbone linkages between
polymer repeat units
Water soluble
Fig. 3.2.7-2 Mechanisms of chemical degradation. Mechanism I involves the cleavage of degradable cross-links between water-soluble
polymer chains. Mechanism II involves the cleavage or chemical transformation of polymer side chains, resulting in the formation of
charged or polar groups. The presence of charged or polar groups leads then to the solubilization of the intact polymer chain. Mechanism
III involves the cleavage of unstable linkages in the polymer backbone, followed by solubilization of the low-molecular-weight fragments.