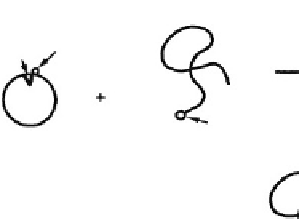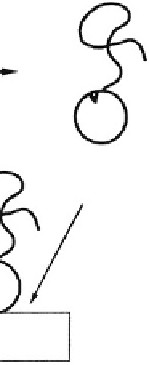Biomedical Engineering Reference
In-Depth Information
polymer was conjugated to specific sites on an endo-
cellulase, which provided on-off control of the enzyme
activity with either light or temperature (
Shimoboji
et al.
, 2001
, 2002a, b, 2003).
Triggered release of bound ligands by the smart
polymer-engineered protein bioconjugates could be used
to release therapeutics, such as for topical drug delivery
to the skin or mucosal surfaces of the body, and also for
localized delivery of drugs within the body by stimulated
release at pretargeted sites using noninvasive, focused
stimuli, or delivery of stimuli from catheters. Triggered
release could also be used to release and recover affinity-
bound ligands from chromatographic and other supports
in eluate-free conditions, including capture and release of
specific cell populations to be used in stem cell and bone
marrow transplantation. These processes could involve
two different stimulus-responsive polymers with sensi-
tivities to the same or different stimuli. For delicate
target ligands such as peptides and proteins, recovery
could be affected without the need for time-consuming
and harsh elution conditions. Triggered release could also
be used to remove inhibitors, toxins, or fouling agents
from the recognition sites of immobilized or free en-
zymes and affinity molecules, such as those used in bio-
sensors, diagnostic assays, or affinity separations. This
could be used to ''regenerate'' such recognition proteins
for extended process use. Light-controlled binding and
release of site-specific protein conjugates may be utilized
as a molecular switch for various applications in bio-
technology, medicine, and bioelectronics, including
hand-held diagnostic devices, biochips, and lab-on-a-chip
devices.
Fong, Stayton, and Hoffman (
Fong
et al.
, 1999
) have
developed an interesting construct to control the dis-
tance of the PNIPAAm from the active site. For this
purpose, they conjugated one sequence of complemen-
tary nucleotides to a specific site near the binding pocket
of streptavidin, and a second sequence to the end of
a PNIPAAm chain. Then, by controlling the location
and length of the complementary sequence, the self-
assembly via hybridization of the two single-chain DNA
sequences could be used to control the distance of the
polymer from the streptavidin binding site.
Genetically-engineered
cystine
−
SH
Binding site
SH reactive group
−
Genetically-engineered
recognition protein
End-reactive
“smart” polymer
Site-specific
polymer-protein
conjugate
Bind to
solid
support
Site-specific polymer-protein
conjugate immobilized on a
solid support
Solid support
Fig. 3.2.6-5 Schematic illustration of the process for preparing an
immobilized, site-specific conjugate of a smart polymer with
a genetically-engineered, mutant protein (Hoffman et al., Journal
of Biomedical Materials Research
2000).
functioning of the protein, or nearby or even within the
active site, in order to control the ligand-protein recog-
nition process and the biological activity of the protein
(
Fig. 3.2.6-4
)(
Ding
et al.
, 1999
, 2001;
Bulmus
et al.
,
1999; Stayton
et al.
, 2000; Shimoboji
et al.
, 2001
, 2002a,
b, 2003). The latter has been most studied by the
Stayton/Hoffman group. Temperature-, pH-, and light-
sensitive smart polymers have been used to form such
novel, ''doubly smart'' bioconjugates. Since the objective
is to control the activity of the protein, and not to phase
separate it, these smart polymer-engineered protein
bioconjugates have usually been immobilized on the
surfaces of microbeads or nanobeads. Stayton, Hoffman,
and co-workers have used such beads in microfluidic
devices for immunoassays (
Malmstadt
et al.
, 2003
).
Earlier work by Hoffman and co-workers established the
importance of matching the smart polymer composition
with the surface composition in order to enhance the
stimulus-driven adsorption of the smart polymer on the
surface (
Miura
et al.
, 1994
). Others have also recently
utilized this phenomenon in microfluidic devices (
Huber
et al.
, 2003
).
The proteins that have been most studied by the
Stayton/Hoffman group to date include streptavidin
and the enzyme cellulase. PNIPAAm-streptavidin site-
specific bioconjugates have been used to control access of
biotin to its binding site on streptavidin, and have enabled
separation of biotinylated proteins according to the size
of the protein (
Ding
et al.
, 2001
). Ding, Stayton, and
Hoffman
et al.
(1999)
also found that raising the tem-
perature to thermally induce the collapse of the polymer
''triggered'' the release of the bound biotin molecules
(
Ding
et al.
, 1999
). For the site-specific enzyme conju-
gates,
Smart polymer hydrogels
When a smart polymer is cross-linked to form a gel, it
will collapse and re-swell in water as a stimulus raises or
lowers it through its critical condition. PNIPAAm gels
have been extensively studied, starting with the
pioneering work of Toyoichi Tanaka in 1981 (
Tanaka,
1981
). Since then, the properties of PNIPAAm hydro-
gels have been widely investigated in the form of beads,
slabs, and multilamellar laminates (
Park and Hoffman,
a
combined
temperature-
and
light-sensitive