Biomedical Engineering Reference
In-Depth Information
3.2.3 Silicone biomaterials:
history and chemistry
Table 3.2.3-1 Key milestones in the development of silicone
chemistry
1824
Berzelius discovers silicon by the reduction of
potassium fluorosilicate with potassium: 4K þ
K
2
SiF
6
/
Si þ 6KF. Reacting silicon with chlorine
gives a volatile compound later identified as
tetrachlorosilane, SiCl
4
:Siþ 2Cl
2
/
SiCl
4
.
Andr
´
Colas and Jim Curtis
Chemical structure
and nomenclature
1863
Friedel and Craft synthesize the first silicon organic
compound, tetraethylsilane: 2Zn(C
2
H
5
)
2
þ SiCl
4
/
Si(C
2
H
5
)
4
þ 2ZnCl
2
.
1871
Ladenburg observes that diethyldiethoxysilane,
(C
2
H
5
)
2
Si(OC
2
H
5
)
2
, in the presence of a diluted acid
gives an oil that decomposes only at a ''very high
temperature.''
Silicones are a general category of synthetic polymers
whose backbone is made of repeating silicon to oxygen
bonds. In addition to their links to oxygen to form the
polymeric chain, the silicon atoms are also bonded to
organic groups, typically methyl groups. This is the basis
for the name ''silicones,'' which was assigned by Kipping
based on their similarity with ketones, because in most
cases, there is on average one silicone atom for one
oxygen and two methyl groups (Kipping, 1904). Later, as
these materials and their applications flourished, more
specific nomenclature was developed. The basic re-
peating unit became known as ''siloxane'' and the most
common silicone is PDMS.
1901-1930s
Kipping lays the foundation of organosilicon
chemistry with the preparation of various silanes by
means of Grignard reactions and the hydrolysis of
chlorosilanes to yield ''large molecules.'' The
polymeric nature of the silicones is confirmed by
the work of Stock.
1940s
In the 1940s, silicones become commercial
materials after Hyde of Dow Corning demonstrates
the thermal stability and high electrical resistance
of silicone resins, and Rochow of General Electric
finds a direct method to prepare silicones from
silicon and methylchloride.
(1996), Rochow (1987), and Noll (1968)dare summa-
rized in
Table 3.2.3-1
.
Many other groups, e.g., phenyl, vinyl and trifluor-
opropyl, can be substituted for the methyl groups along
the chain. The simultaneous presence of ''organic'' groups
attached to an ''inorganic'' backbone gives silicones
a combination of unique properties, making possible
their use as fluids, emulsions, compounds, resins, and
elastomers in numerous applications and diverse fields.
For example, silicones are common in the aerospace in-
dustry, due principally to their low and high temperature
performance. In the electronics field, silicones are used as
electrical insulation, potting compounds and other ap-
plications specific to semiconductor manufacture. Their
long-term durability has made silicone sealants, adhe-
sives and waterproof coatings commonplace in the con-
struction industry. Their excellent biocompatibility
makes many silicones well suited for use in numerous
personal
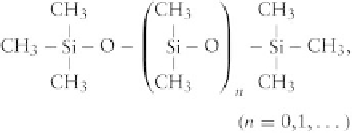
Nomenclature
The
most
common
silicones
are
the
poly-
dimethylsiloxanes
trimethylsilyloxy
terminated,
with
the following structure:
These are linear polymers and liquids, even for large
values of
n.
The main chain unit, -(Si(CH
3
)
2
O)
n
-, is
often represented by the letter D because, as the silicon
atom is connected with two oxygen atoms, this unit is
capable of expanding within the polymer in two di-
rections. M, T and Q units are defined in a similar
manner, as shown in
Table 3.2.3-2
.
The system is sometimes modified by the use of su-
perscript letters designating nonmethyl substituents, for
example, D
H
¼
H(CH
3
)SiO
2/2
and M
f
or M
Ph
¼
(CH
3
)
2
(C
6
H
5
)SiO
1/2
(Smith, 1991). Further examples
are shown in
Table 3.2.3-3
.
care,
pharmaceutical,
and
medical
device
applications.
Historical milestones in silicone chemistry
Key milestones in the development of silicone chemis-
tryd thoroughly described elsewhere by Lane and Burns