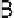Biomedical Engineering Reference
In-Depth Information
A
B
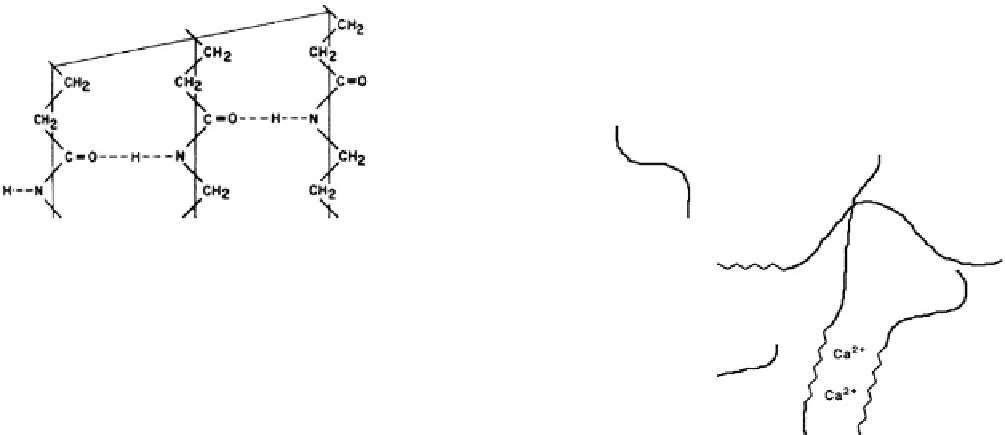
Fig. 3.2.2-7 (A) Hydrogen bonding in nylon-6,6 molecules in a triclinic unit cell:
s
form. (From L. Mandelkern, An Introduction to
Macromolecules, Springer-Verlag, 1983, p. 43, with permission.) (B) Ionic aggregation giving rise to physical cross-links in copolymers.
Mechanical properties
structure resulting from chemical cross-links and chain
entanglements prevents large-scale movement or flow.
Thus, rubbery polymers tend to exhibit a lower modulus,
or stiffness, and extensibilities of several hundred per-
cent, as shown in
Table 3.2.2-1
. Rubbery materials may
also exhibit an increase of stress prior to breakage as
a result of strain-induced crystallization assisted by mo-
lecular orientation in the direction of stress. Glassy and
semicrystalline polymers have higher moduli and lower
extensibilities.
The ultimate mechanical properties of polymers at
large deformations are important in selecting particular
The tensile properties of polymers can be characterized
by their deformation behavior (stress-strain response
(
Fig. 3.2.2-9
). Amorphous, rubbery polymers are soft
and reversibly extensible. The freedom of motion of the
polymer chain is retained at a local level while a network
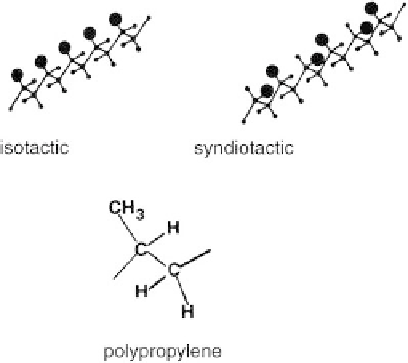
Fig. 3.2.2-8 Schematic of stereoisomers of PP. (From
F. Rodriguez, Principles of Polymer Systems, Hemisphere Publ.,
1982, p. 22, with permission.)
Fig. 3.2.2-9 Tensile properties of polymers.