Biomedical Engineering Reference
In-Depth Information
Water solvent properties
dated water-structure concepts will not be discussed
further here, other than to caution the reader that the
transient nature of hydrogen bonding greatly weakens the
notion of a ''structure'' as it might be practically applied
by a chemist for example (
Berendsen, 1967
) and that
reference to water structure near solutes and surfaces in
terms of ''icebergs'' or ''melting'' should not be taken too
literally, as will be discussed further subsequently.
A very important chemical outcome of this propensity
of water to self-associate is the dramatic effect on water
solvent properties. One view of self-association is from
the standpoint of Lewis acidity and basicity. It may be
recalled from general chemistry that a Lewis acid is
a molecule that can accept electrons or, more generally,
electron density from a molecular orbital of a donor
molecule. An electron-density donor molecule is termed
a Lewis base. Water is amphoteric in this sense because,
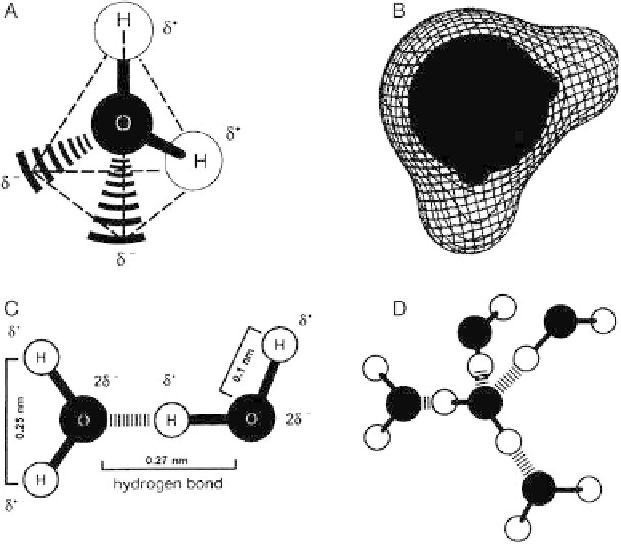
as illustrated in
Figs. 3.1.5-1
A and
3.1.5-1
D, it can
simultaneously share and donate electron density.
Hydrogen atoms (the Lewis acids) on one or more ad-
jacent water molecules can accept electron density from
the unshared electron pairs on the oxygen atom (the
Lewis bases) of another water molecule. In this manner,
water forms a 3D network through Lewis acid-base self-
association reactions.
If the self-associated network is more complete than
some arbitrary reference state, then there must be
Figures 3.1.5-1A-3.1.5-1D
collect various diagrams of
water illustrating the familiar atomic structure and how
this arrangement leads to the ability to form a network of
self-associated molecules through hydrogen bonding.
Self-association confers unique properties on water,
many of which are still active areas of scientific in-
vestigation even after more than 200 years of chemical
and physical research applied to water (
Franks, 1972
).
Hydrogen bonds in water are relatively weak 3-5 kcal/
mole associations with little covalent character (
Iassacs
et al.
, 1999; Marshall, 1999
). As it turns out, hydrogen
bond strength is approximately the same as the energy
transferred from one molecule to another by collisions at
room temperature (
Vinogradov and Linnell, 1971
). So
hydrogen bonds are quite transient in nature, persisting
only for a few tens of picoseconds (
Berendsen, 1967;
Luzar and Chandler, 1996
). Modern molecular simula-
tions suggest, however, that more than 75% of liquid-
water molecules are interconnected in a 3D network of
three or four nearest neighbors at any particular instant in
time (
Robinson
et al.
, 1996
). This stabilizing network of
self-associated water formed from repeat units illus-
trated in
Fig. 3.1.5-1
D is so extensive, that it is fre-
quently termed ''water structure,'' especially in the older
literature (
Narten and Levy, 1969
). These somewhat
Fig. 3.1.5-1 Atomic structure of water illustrating (A) tetrahedral bonding arrangement wherein hydrogen atoms (H, light-colored
spheres) are Lewis acid centers and the two lone-pair electrons on oxygen (O, dark-colored spheres) are Lewis base centers that
permit water to hydrogen bond with four nearest-neighbor water molecules; (B) electron density map superimposed on an atomic-radius
sphere model of water providing a more authentic representation of molecular water; (C) approximate molecular dimensions; and (D)
five water molecules participating in a portion of a hydrogen-bond network.