Biomedical Engineering Reference
In-Depth Information
6000
Table 3.1.4-5 Analytical capabilities of SIMS
C-O
5000
Static
SIMS
Dynamic
SIMS
4000
Identify hydrogen and deuterium
UU
3000
C-C and C-H
Identify other elements
(often must be inferred from the data)
UU
O=C-NH
2
2000
Suggest molecular structures
(inferred from the data)
-
U
1000
O=C-OR
Observe extremely high mass fragments
(proteins, polymers)
-
U
0
294
292
290
288
286
284
282
280
Detection of extremely low concentrations
UU
Binding energy (eV)
)
Depth profile to 1 mm into the sample
U
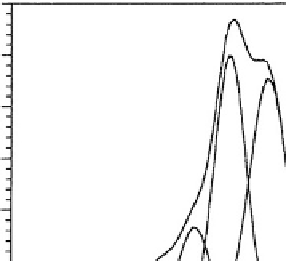
Fig. 3.1.4-9 The carbon 1s narrow scan ESCA spectrum of
a surface-modified PMMA ophthalmologic device. Narrow scan
spectra can be generated for each element seen in low-energy
resolution mode in Fig. 3.1.4-8.
Observe the outermost 1-2 atomic layers
UU
High spatial resolution
(features as small as
w
400
˚
)
UU
Semiquantitative analysis
(for limited sets of specimens)
UU
the peak position in the narrow scan of the sulfur region
(S2p spectrum) suggests sulfonate-type groups. The shape
of the C1s spectrum, the position of the sulfur peak, and
the presence of nitrogen all suggest that heparin was
immobilized to the surface of the PMMAdevice. Since the
stoichiometry of the lens surface does not match that for
pure heparin, this suggests thatwe are seeing either some of
the PMMA substrate through a
<
100
˚
layer of heparin, or
we are seeing some of the bonding links used to immobilize
the heparin to the lens surface. Further ESCA analysis will
permit the extraction of more details about this surface-
modified device, including an estimate of surface modifi-
cation thickness, further confirmation that the coating is
indeed heparin, and additional information about the
nature of the immobilization chemistry.
Useful for polymers
-
U
Useful for inorganics
(metals, ceramics, etc.)
UU
Useful for powders, films,
fibers, etc.
UU
*
Cluster ion sources may allow depth profiling with static SIMS-like information
content
enough energy to them so they sputter from the surface
into the vacuum phase. The process is analogous to racked
pool balls that are ejected from the cluster by the impact
of the cue ball; the harder the cue ball hits the rack of balls,
themore balls are emitted fromthe rack. In SIMS, the cue
balls are ions (xenon, argon, cesium, and gallium ions are
commonly used) that are accelerated to energies of 5000-
20,000 eV. The particles ejected from the surface are
positive and negative ions (secondary ions), radicals, ex-
cited states, and neutrals. Only the secondary ions are
measured in SIMS. In ESCA, the energy of emitted par-
ticles (electrons) ismeasured. SIMSmeasures themass of
emitted ions (more rigorously, the ratio of mass to charge,
m/z)
using a time-of-flight (TOF) mass analyzer or
a quadrupole mass analyzer.
There are two modes for SIMS analysis, depending on
the ion flux: dynamic and static. Dynamic SIMS uses high
ion doses in a given analysis time. The primary ion beam
sputters somuchmaterial fromthe surface that the surface
erodes at an appreciable rate. We can capitalize on this to
do a depth profile into a specimen. The intensity of the
m/z
peak of a species of interest (e.g., sodium ion,
m/z ¼
23)
Secondary ion mass spectrometry
Secondary ion mass spectrometry (SIMS) is an important
addition to the armamentarium of tools that the surface
analyst can bring to bear on a biomedical problem. SIMS
produces a mass spectrum of the outermost 10
˚
of a sur-
face. Like ESCA, it requires complex instrumentation and
an ultrahigh vacuum chamber for the analysis. However, it
provides unique information that is complementary to
ESCA and greatly aids in understanding surface composi-
tion. Some of the analytical capabilities of SIMS are sum-
marized in
Table 3.1.4-5
. Review articles on SIMS are
available (Ratner, 1983;
Scheutzle
et al.
, 1984; Briggs, 1986;
Davies and Lynn, 1990; Vickerman
et al.
, 1989; Benning-
hoven, 1983; Van Vaeck
et al.,
1999; Belu
et al
., 2003
).
In SIMS analysis, a surface is bombarded with a beam
of accelerated ions. The collision of these ions with the
atoms and molecules in the surface zone can transfer