Biomedical Engineering Reference
In-Depth Information
that are tightly packed and firmly bound together. This is
the microstructure of the material that is observed at
magnifications where the resolution is between 1-100
m
m.
In pure elemental materials, all the crystals have the
same structure and differ from each other only by virtue of
their different orientations. In general, these crystallites or
grains are too small to be seen except with a light micro-
scope. Most solids are opaque, however, so the common
transmission (biological) microscope cannot be used. In-
stead, a metallographic or ceramographic reflecting mi-
croscope is used. Incident light is reflected from the
polished metal or ceramic surface. The grain structure is
revealed by etching the surface with a mildly corrosive
medium that preferentially attacks the grain boundaries.
When this surface is viewed through the reflecting
microscope the size and shape of the grains, i.e., the mi-
crostructure, is revealed.
Grain size is one of the most important features that
can be evaluated by this technique because fine-grained
samples are generally stronger than coarse-grained speci-
mens of a given material. Another important feature that
can be identified is the coexistence of two or more phases
in some solid materials. The grains of a given phase will all
have the same chemical composition and crystal struc-
ture, but the grains of a second phase will be different in
both these respects. This never occurs in samples of pure
elements, but does occur in mixtures of different ele-
ments or compounds where the atoms ormolecules can be
dissolved in each other in the solid state just as they are in
a liquid or gas solution.
For example, some chromium atoms can substitute for
iron atoms in the FCC crystal lattice of iron to produce
stainless steel, a solid solution alloy. Like liquid solutions,
solid solutions exhibit solubility limits; when this limit is
exceeded, a second phase precipitates. For example, if
more Cr atoms are added to stainless steel than the FCC
lattice of the iron can accommodate, a second phase that is
chromium rich precipitates. Many important biological
and implant materials are multiphase (
Hummel, 1997
).
These include the cobalt-based and titanium-based or-
thopedic implant alloys and the mercury-based dental
restorative alloys, i.e., amalgams.
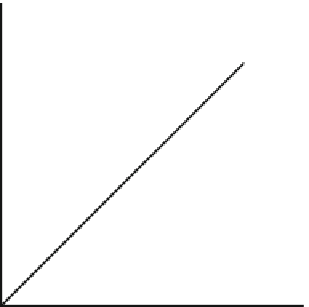
Load
(newtons)
Extension (mm)
Fig. 3.1.2-3 Initial extension is proportional to load according to
Hooke's law.
mechanical properties on microstructure is so great that
it is one of the fundamental objectives of materials sci-
ence to control mechanical properties by modifying
microstructure.
Elastic behavior
The basic experiment for determining mechanical
properties is the tensile test. In 1678, Robert Hooke
showed that a solid material subjected to a tensile (dis-
traction) force would extend in the direction of traction
by an amount that was proportional to the load
(
Fig. 3.1.2-3
). This is known as Hooke's law and simply
expresses the fact that most solids behave in an elastic
manner (like a spring) if the loads are not too great.
Stress and strain
The extension for a given load varies with the geometry
of the specimen as well as its composition. It is, there-
fore, difficult to compare the relative stiffness of differ-
ent materials or to predict the load-carrying capacity of
structures with complex shapes. To resolve this confu-
sion, the load and deformation can be normalized. To do
this, the load is divided by the cross-sectional area
available to support the load, and the extension is divided
by the original length of the specimen. The load can then
be reported as load per unit of cross-sectional area, and
the deformation can be reported as the elongation per
unit of the original length over which the elongation oc-
curred. In this way, the effects of specimen geometry can
be normalized.
The normalized load (force/area) is stress (s) and the
normalized deformation (change
Mechanical properties of materials
Solid materials possess many kinds of properties (e.g.,
mechanical, chemical, thermal, acoustical, optical, elec-
trical, magnetic). For most (but not all) biomedical ap-
plications, the two properties of greatest importance are
strength (mechanical) and reactivity (chemical). The
remainder of this section will be devoted to mechanical
properties, their measurement, and their dependence on
structure. It is significant to note that the dependence of
in length/original
length) is strain (3)(
Fig. 3.1.2-4
).