Biomedical Engineering Reference
In-Depth Information
electrons of the atoms are now tightly held in the locality
of the ionic bond. These bound electrons are no longer
available to serve as charge carriers and ionic solids are
poor electrical conductors. Finally, the low overall energy
state of these substances endows themwith relatively low
chemical reactivity. Sodium chloride (NaCl) and mag-
nesium oxide (MgO) are examples of ionic solids.
A
B
Covalent bonding
C
D
Elements that fall along the boundary between metals
and nonmetals, such as carbon and silicon, have atoms
with four valence electrons and about equal tendencies to
donate and accept electrons. For this reason, they do not
form strong ionic bonds. Rather, stable electron struc-
tures are achieved by sharing valence electrons. For ex-
ample, two carbon atoms can each contribute an electron
to a shared pair. This shared pair of electrons constitutes
j
j
the covalent bond
C
C
(
Hummel, 1997
).
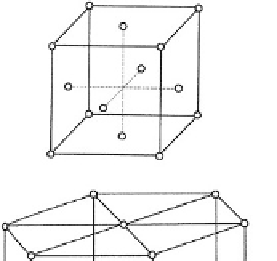
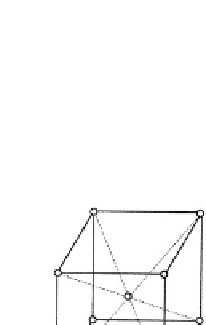
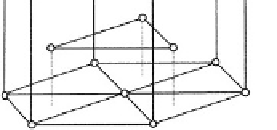
Fig. 3.1.2-1 Typical metal crystal structures (unit cells). (A) Face-
centered cubic (FCC). (B) Full size atoms in FCC. (C) Hexagonal
close-packed (HCP). (D) Body-centered cubic (BCC).
j
j
If a central carbon atom participates in four of these
covalent bonds (two electrons per bond), it has achieved
a stable outer shell of eight valence electrons. More carbon
atoms can be added to the growing aggregate so that every
atom has four nearest neighbors with which it shares one
bond each. Thus, in a large grouping, every atomhas a stable
electron structure and four nearest neighbors. These
neighbors often form a tetrahedron, and the tetrahedra in
turn are assembled in an orderly repeating pattern (i.e.,
a crystal) (
Fig. 3.1.2-2
). This is the structure of both di-
amond and silicon. Diamond is the hardest of all materials,
which shows that covalent bonds can be very strong. Once
again, the bonding process results in a particular electronic
structure (all valence electrons in pairs localized at the
metal) and an anion (e.g., nonmetal), which are strongly
attracted by the electrostatic or Coulomb effect. This
attraction of cations and anions constitutes the ionic bond
(
Hummel, 1997
).
In ionic solids composed of many ions, the ions are
arranged so that each cation is surrounded by as many
anions as possible to reduce the strong mutual repulsion
of cations. This packing further reduces the overall energy
of the assembly and leads to a highly ordered arrangement
called a crystal structure (
Fig. 3.1.2-1
). Note that in such
a crystal no discrete molecules exist, but only an orderly
collection of cations and anions. The loosely bound
Fig. 3.1.2-2 Crystal structures of carbon. (A) Diamond (cubic). (B) Graphite (hexagonal).