Biomedical Engineering Reference
In-Depth Information
A critical component of the early clinical development studies is the study design.
When study design is donewell, it will minimize the effects of preanalytical sources of
variability, such as the effects of posture, exercise, diurnal biorhythms, food intake,
and so on. If the preanalytical variability has not been characterized in advance of the
clinical studies, it is usually necessary to obtain several samples prior to dosing to
better understand the intrasubject variability. The early clinical studies are ideal for
determining intrasubject variability as some of the subjects are treated with placebo.
The intrasubject variability in the measured biomarker will describe the total
variability, which consists of the sum of the analytical, preanalytical, and biological
variability. Knowledge of the intrasubject variability will ultimately determine the
number of subjects needed in a clinical study to statistically observe a certain change
in the biomarker and also to generate data that can support decision making.
There is often a delay between the drug exposure and the PD effect, which usually
means that samples for biomarker measurements need to be collected well beyond
the expected T
max
(time of maximum drug concentration) for the drug. Sometimes the
PD effect will last for days despite a relatively short half-life of the drug and this
is especially true for irreversible inhibitors. An example of this is aspirin that has
a half-life of about 17 min, but despite this 1.0 g of aspirin (Figure 3.3) will cause an
inhibition of platelet thromboxane A
2
for up to 7-10 days [34]. In another example,
increases in gene expression of ABCA1 and ABCG1 occurred approximately 2-3 h
after the T
max
of the drug LXR-623, a novel liver X-receptor agonist [35].
Another challenge for study design is a biomarker that shows significant diurnal
variation. An example of this is plasma 7-
-hydroxy-4-cholesten-3-one, which
actually shows two distinctive peaks (two- to fourfold above baseline) during a
a
500
400
300
200
100
0
0-1.5
1.5-3.0
3.0-4.5
4.5-6.0
6.0-7.5
Time Interval (h)
FIGURE 3.3
Urinary excretion of thromboxane (measured as the urinary metabolite
2,3-dinor-TxB
2
) following a single dose of 1.0 g of aspirin (Adapted from Ref. 40, with
permission).
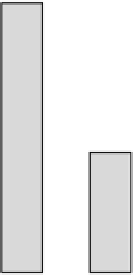

















Search WWH ::

Custom Search