Biomedical Engineering Reference
In-Depth Information
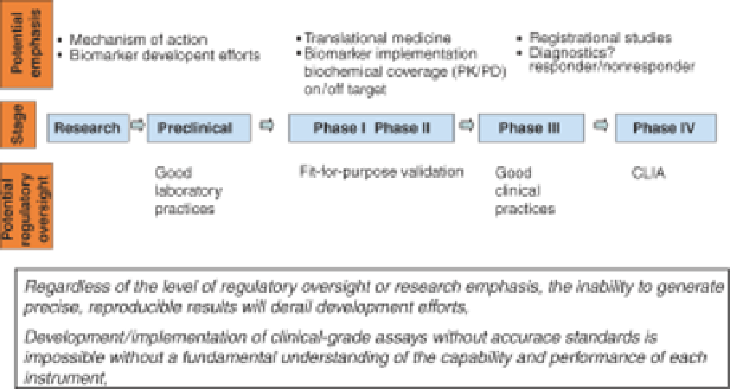
FIGURE 13.1
The versatility of flow cytometers makes them an ideal platform for integration
into the biopharmaceutical drug development process. It is important to calibrate administrative
oversight to each stage of drug development. This is key with respect to instrument validation
where GLP studies place the highest demand on validation activities. Given the intensive nature
of GLP validation exercises, it would be inadvisable to apply this guidance to other stages of the
development process; however, the core principles of GLP-driven instrument validation are of
value throughout the development and commercialization scheme (see text for discussion).
validations are often cited, a question of relevance is whether any common denomi-
nator can be gleaned from efforts with the CLIA, the GLPs, or the GCPs or whether
these concepts introduce unnecessary cost. Thus, the roadmap in deciding how a flow
cytometer should be used in a validated setting starts with the basic knowledge of
knowing where you are in the regulated world (Figure 13.1).
13.2 ATTRIBUTES OF CLIA, GLP, AND GCP INSTRUMENT
VALIDATIONS
In industry, it is not unusual for a team to be charged with a service function for
specimens derived from a variety of sources/studies. Activities need not be segre-
gated, and work stream amalgamation represents a value-oriented strategy because
infrastructural needs are minimized through efficiency. In other words, if a laboratory
has only a 20%need for CLIA-governed analyses, the excess capacity could host GLP
or GCP studies provided that efforts are appropriately defined.
13.2.1 CLIA
When human specimens are analyzed to provide diagnostic information, to guide
treatment decisions during disease, or to perform health assessments, the laboratory is
Search WWH ::

Custom Search