Biomedical Engineering Reference
In-Depth Information
(a)
(b)
0.4
0.4
0.3
0.3
0.2
0.2
0.1
0.1
0.0
0.0
% Uptake
% Uptake
(c)
(d)
0.4
0.4
0.3
0.3
0.2
0.2
0.1
0.1
0.0
0.0
% Uptake
% Uptake
FIGURE 10.6
Effect of human serum matrix on normal frequency distribution at various
minimum required dilutions: 1:10 (a), 1:20 (b), 1:40 (c), and 1:80 (d).
control antibody with a fixed amount of the drug-fluorochrome conjugate
(Figure 10.5c). Biological matrix is added to the assay system and the effect observed.
Figure 10.6 shows the effect of diluting human serum, 1/10, 1/20, 1/40, and 1/80, and
the frequency distribution of percent uptake of fluorescently labeled drug observed at
the various dilutions. This figure demonstrates the minimum required dilution (MRD)
of the human serum matrix to achieve a normal distribution of response was 1/40.
Throughout all phases of an assay's life cycle, reliable and sustainable sources of
critical assay reagents are pivotal to the success of the nonclinical or clinical program
that the assay supports.
10.3.1
Instrument Differences
Assays using GMFI signal output from flow cytometers are susceptible to complex-
ities of the instrument, such as laser strength and alignment, instrument fluidics, and
rigors of instrument maintenance. Due to the complexities of the instruments, the
variation between instruments can be up to 50% and thus operational strategies need
to be considered and outlined for longitudinal studies. During assay validation,
signal output as a function of an instrument can be assessed using the same
samples to compare different instruments, if available. For assays where changing




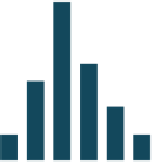






































































Search WWH ::

Custom Search