Biomedical Engineering Reference
In-Depth Information
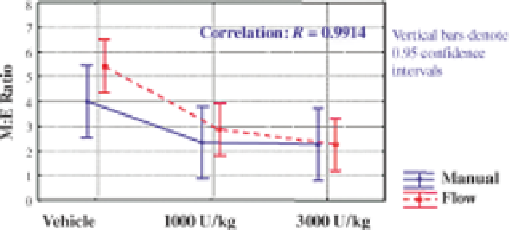
FIGURE 6.7 Comparison of M:E ratio methods in mice dosed with recombinant human
erythropoietin. Mice were dosed with vehicle, 1000 U/kg or 3000 U/kg rhEpo. Bone marrow
differentials and M:E ratios were measured by flow cytometric and cytologic methods. Graph
presents results from a repeated measure analysis of variance (ANOVA) model.
example, in order to be characterized as a lymphocyte a cell must stain positively for
CD45 but negatively for both CD11b and ter119. There is little chance for
investigator bias to negatively impact cell determination on a cell-by-cell basis,
whereas manual cytologic determination is based solely on a single medical
technologist
s judgment for each individual cell. In addition, flow cytometry has
the advantage of decreased absolute counting errors due to the large number of cells
interrogated (at least 10,000) versus only 100-500 for manual methods. Therefore, a
case could easily be made as to why the flow cytometry results would be more
accurate and reliable. However, the reality is that manual cytologic assessment is the
“Gold Standard” and the incongruence observed simply cannot be dismissed.
Possible explanations for this incongruence, other than stating that one method is
right and the other one iswrong, do exist. The first is that manual and flowcytometric
analyses were not performed on identical samples. Flow cytometric analysis was
performed on suspensions of bone marrow cells from the left femur and cytology
was performed on a smear created from the right femur. However, previous
experiments did not reveal significant differences between femurs. Instead, differ-
ences may be attributed to the fact that smears are created froma small portion of the
total femoral bone marrow, whereas flow cytometric preparation homogenizes the
entire femoral bone marrow population, precluding potential sampling errors. A
second possible explanation for lack of parity is the fact that some events are
excluded from analysis during flow cytometric assessment. This is necessitated by
the existence of minor populations of cell aggregates and nonstaining cells.
Although the nonstaining population does not stain positively for any of the
monoclonal antibodies used, it does stain with the nuclear dye, Hoechst 33342.
This staining pattern argues that the events are not hematopoietic lineage cells and
should, in fact, be excluded from analysis. The identity of these events could
be primitive stem cells that do not yet express lineage markers, other resident bone
marrow cell types (e.g., osteoblasts, osteoclasts, and osteocytes), or perhaps free
nuclei. It is tempting to speculate that these events are free nuclei of erythroid cells
that were in the process of nuclear extrusion upon harvest, but there is no direct
Search WWH ::

Custom Search