Biomedical Engineering Reference
In-Depth Information
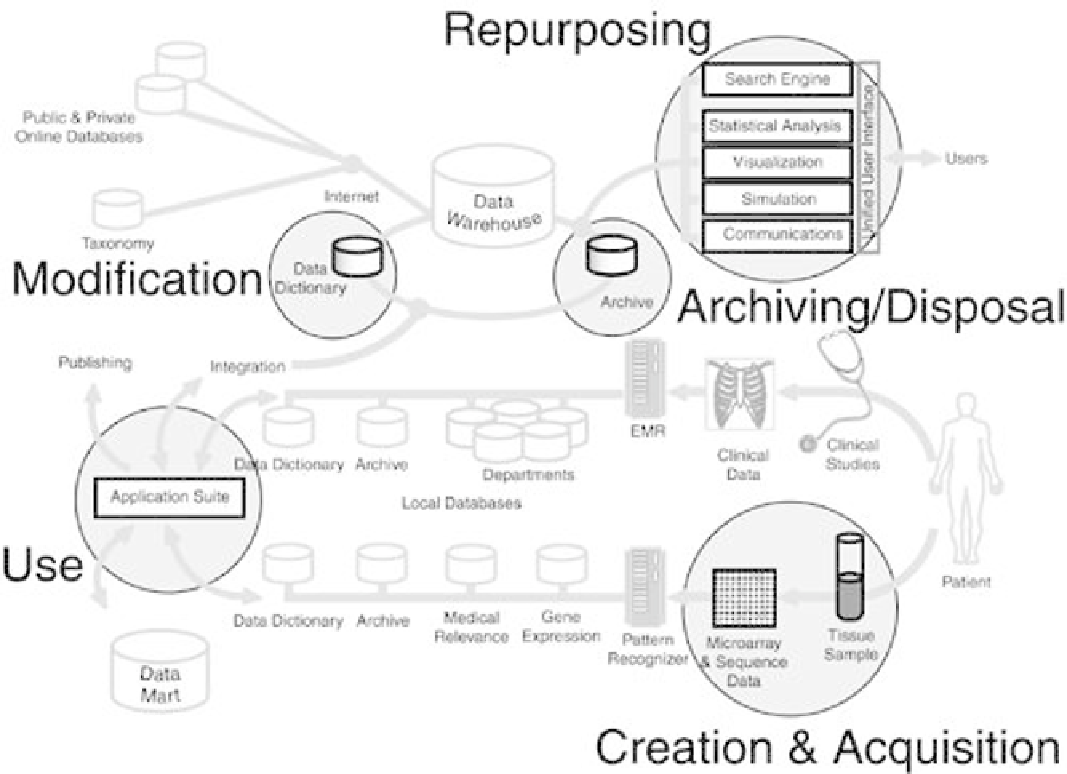
Data Life Cycle
In the data-management process, data are authored by clinicians and researchers and generated
directly by research and test equipment, used by a variety of applications, repurposed or modified for
other uses, and archived for future study. Eventually, the data are disposed of, freeing the data
warehouses and other hardware from the overhead of maintaining low-value data. The overall
process, from data creation to disposal, is normally referred to as the data life cycle, as depicted in
Figure 2-8
. The highlights of each stage are described there.
Figure 2-8. Data Life Cycle. Key steps in the process include data creation
and acquisition, use, modification, repurposing, and the end
game—archiving and disposal. The same process applies to data in a
desktop workstation or, as in this illustration, to a large pharmacogenomic
operation with multiple, disparate systems.
Data Creation and Acquisition
The process of data creation and acquisition is a function of the source and type of data. For
example, in the scenario depicted in
Figure 2-8
, data are generated by sequencing machines and
microarrays in the molecular biology laboratory, and by clinicians and clinical studies in the clinic or
hospital. Depending on the difficulty in creating the data and the intended use, the creation process
may be trivial and inexpensive or extremely complicated and costly. For example, recruiting test
subjects to donate tissue biopsies is generally more expensive and difficult than identifying patients
who are willing to provide less-invasive (and painful) tissue samples.