Geoscience Reference
In-Depth Information
70
SO
2
NO
2
NH
3
60
50
40
30
20
10
0
1880
1910
1940
1970
2000
2030
Year
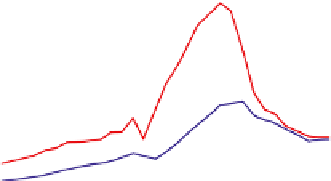
Figure 7.1
The rise and fall of emissions of sulphur and nitrogen in Europe over the
period 1880-2030 as estimated by Schöpp
et al
. (2003). Units are Mt yr
−1
of SO
2
(red
line), NO
2
(blue line) and NH
3
(green line), respectively. Estimates for the future (2000-
30) assume full implementation of current legislation (CLe scenario).
conducted in the 1990s indicated that about 10% of all fish stocks in western
Scandinavia's 126,000 lakes had been affected by acidification (Tammi
et al
.
2003). The situation in Norway has been quite dramatic, with lakes in 30% of the
country suffering damage to fish populations (Hesthagen
et al
. 1999). The story
has been similar in other parts of Europe and in eastern North America, with
extensive acidification reported from south-eastern Canada and upland areas of
eastern United States. Here also, S emissions peaked in the 1980s. Inorganic
aluminium and hydrogen ions are the primary agents of toxicity. The strong acid
anions, sulphate, nitrate and chloride, bring these acid cations into soil solution
and to runoff. The links between emissions of air pollutants, deposition of S and
N, acidification of surface waters and damage to aquatic organisms thus lie in
following the fates of S, N and, to a lesser extent, chloride (Cl).
Two factors are needed for surface water acidification: the water must be acid-
sensitive and the area must receive sufficient amounts of acid deposition (Wright &
Henriksen 1978). Acid sensitive lakes and streams are found throughout the
world in catchments with weathering-resistant bedrock such as granite and
quartzite and young, often poorly-developed podsolic and organic-rich soils
(Skjelkvåle & Wright 1990). In these waters, the dominant inorganic anion is
usually the weak-acid anion bicarbonate (HCO
3
), whose source is respiration by
plant roots and dissolution in soil water. HCO
3
is generally accompanied by the
base cations, calcium (Ca) and magnesium (Mg), and the concentrations of all
three depend on the ease by which the soil minerals can be broken down by
weathering. Acid-sensitive waters have low concentrations of these ions.
The second factor is the amount of acid deposition. The most sensitive waters
are affected when the rain is more acidic than about pH 4.7 and pollutant sulphate
(SO
4
) concentrations exceed about 20
m
eq l
−1
. In acidified waters, the strong-acid
anion SO
4
is usually the dominant ion and replaces HCO
3
. Acidified, SO
4
-rich
waters have pH often below 5 and increased concentrations of inorganic
aluminium species (Al
n
+
). The acid and inorganic Al are toxic to fish and other
aquatic organisms.
























































Search WWH ::

Custom Search