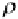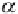Geology Reference
In-Depth Information
or the increase in incoming solar radiation. The solar
radiation factor can be neglected in the case of polar ice
due to the near absence of sunlight during most of the
winter season and the low elevation of the sun during the
rest of the growth period. Otherwise, surface melt abla-
tion must be taken into consideration. This is determined
by surface temperature, which is a strong function of the
radiative flux. For a surface at steady temperature, con-
servation of energy requires that the net radiative flux
Q
n
must be balanced with the heat fluxes plus the heat con-
sumed by surface melt:
typical values of
ρ
a
,
C
s
,
C
e
,
C
p
, and
f
.
Maykut
[1978] gives
daily values of air temperature, incoming short‐wave
and long‐wave radiation in the Arctic based on a polyno-
mial smoothing technique developed by
Maykut and
Untersteiner
[1971].
Edgar and Ackley
[1981] used the above formulation to
determine the meteorological variables responsible for
the difference between the ice surface ablation seasons
in the Arctic and Antarctic. They found that Antarctic ice
rarely exhibits surface ablation (i.e., melt pond) as com-
pared to the Arctic ice. They determined the reason for
this was the low relative humidity associated with the
relatively dry winds off the Antarctic continent as well as
the smaller effective radiation parameters in the Antarctic.
QFFFLdmdt
n
f
(
/
)
(2.19)
c
s
e
Once again,
F
c
is the conductive heat flux through the
bulk of ice and snow. The last term in the right‐hand side
represents the energy consumed by surface melt, where
L
f
is the latent heat of fusion (the energy absorbed when a
substance melts or released when it freezes), and
m
is the
mass of water formed per unit surface area as a result of
melt;
F
s
and
F
e
are the sensible and latent heat, respec-
tively, given by the following expressions:
2.3. InclusIons In Ice
Water is an excellent solvent for many substances in
the air and the atmosphere and this feature is specially
required for the survival and growth of most living organ-
isms. Dissolved salts and gases exist at a much higher
quantity in seawater (inorganic salts constitute about
34‰-35‰ by weight) than in lake or river water (around
1‰). However, when water solidifies to ice, the ice lattice
retains practically nothing. Except for very minute quan-
tities of certain elements and compounds, all the dis-
solved materials in the water are rejected on solidification
when the amorphous state of water changes to a crystal-
line structure.
At ordinary atmospheric pressures and temperature
(say, higher than −70 °C), crystalline structure of ice is
close‐packed hexagonal (Figure 4.4 in Chapter 4). The
oxygen‐oxygen lattice of the hexagonal crystal practically
does not allow any atoms other than oxygen (O) and
hydrogen (H). There are a few exceptions, but their solu-
bility is extremely low. These are a few common acids,
such as HCl and HF, ammonia (NH
3
), a few alkalis
(KOH, NaOH) and their derivatives such as NH
4
F
(NH
3
+ HF). However, brine and gas inclusions are
trapped as pockets in between the ice crystals.
For this reason, sea ice may be considered as a rather
complex porous material consisting of pure ice crystals
and inclusions that can exist in three different phases
depending on its age, thermal state, and history. The
inclusions could be liquid in the form of brine pockets,
gas in the form of bubbles either isolated (separate) or
inside the brine pockets, and solid in the form of precipi-
tated salt crystals. For practical purposes, sea ice can be
considered as a two‐phase (binary) material comprising
of pure ice and one type of dominant inclusion. The
dominant inclusion could be brine pockets with or with-
out air bubbles trapped inside them or air pockets. In case
of new and up to a year old ice, first-year (FY) ice, the
dominant inclusions exist in the form of brine pockets
F CUTT
s
(
)
(2.20)
a sp s
a
(2.21)
FCLUqfq
e
(
)
a ev a
0
where
ρ
a
is the air density,
C
s
is the sensible heat transfer
coefficient,
C
e
is the evaporation coefficient,
C
p
is the spe-
cific heat at constant pressure,
L
v
is the latent heat of
vaporization (in joules per kilogram),
U
is the wind speed
at a reference height (10 m),
T
a
and
T
s
are the air and
surface temperatures,
q
a
and
q
0
are the specific humidity
at 10 m above the surface and at the surface, respectively,
and
f
is the relative humidity. Both
F
c
and
m
depend on
T
s
. If equations (2.20) and (2.21) are substituted into
equation (2.19), then
T
s
can be determined following
a method suggested in
Maykut
[1978]. The net flux in
equation (2.19) can be decomposed into its radiation
components:
(2.22)
Q
(
1
)
F IF F
n
r
0
L
L
where
α
is the ice surface albedo (the ratio of reflected to
the incident solar short‐wave energy),
F
r
is the incoming
short‐wave radiation,
I
0
is the short‐wave flux penetrat-
ing the ice surface, and
F
L
⇓ and
F
L
⇑ are the incoming
and emitted long‐wave radiation, respectively. In the
above equations, the flux direction toward the surface is
considered to be positive.
Cox and Weeks
[1988] present
expressions for
L
v
as a function of air temperature, the
emitted long‐wave radiation in terms of air temperature,
the difference in specific humidity (
q
a
−
q
0
) (which is a
function of both air and surface temperatures),
I
0
(which
is a function of the reflected short‐wave [(1 −
α
)
F
r
], and
α
(which is a function of ice thickness). They also present