Geology Reference
In-Depth Information
4
Temperature of maximum density
Freezing temperature
3
2
1
0
-1
-2
-
24.69 (‰)
-3
-4
02468 0 2 4 6 8
Salinity (‰)
20
22
24
26
28
30
32
34
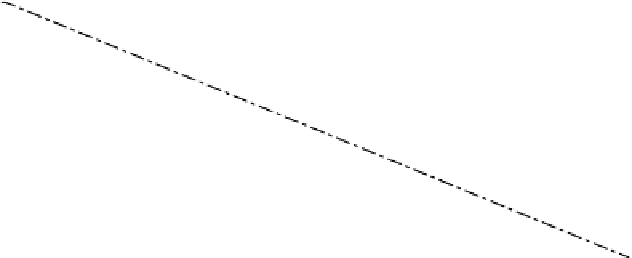
Figure 2.4
Dependence of the temperature of maximum density and the freezing point on water salinity; the two
curves intersect at the salinity of 24.69‰.
It is appropriate to mentioned here that the density
of seawater at the surface of the ocean varies between
1020 and 1029 kg/m
3
depending on the salinity of the
water. Although the density of pure ice is about 917 kg/
m
3
, the density of newly formed sea ice is only marginally
less than 1000 kg/m
3
due to its high brine content.
Nonetheless, it is lighter than the sea water and floats on
the surface. Of course brine in the ice drains very quickly
and makes the ice even lighter.
Air is also dissolved in seawater. Through the constant
stirring of the sea surface by wind and waves, gases are
transferred from the atmosphere to the water. The three
common gases that make up 99.93% of the atmosphere are
nitrogen (78.08%), oxygen (20.95%), and argon (0.93%).
The sum of their total percentages in the dissolved air in
seawater is 98.5% (62.6% nitrogen, 34.3, oxygen, and 1.6%
argon) [
Pilson,
1998]. The solubility of oxygen in water
is relatively higher than nitrogen. This results in a lower
nitrogen content in water and hence ice. Carbon dioxide
represents only 0.035% of atmospheric gases, but it con-
stitutes a relatively larger percentage (1.4%) of the total
air dissolved in seawater. The marine organisms may be a
factor that contributes to the higher CO
2
percentage. As the
temperature or the salinity of seawater increases, the amount
of gas that ocean water can dissolve decreases slightly. When
seawater freezes, air is segregated and entrapped in the
form of pockets along with brine as the pockets at the
intercrystalline boundaries.
of a nucleus around which ice crystals can form. In case of
natural water bodies nuclei could be dust particles, snow-
flakes, frozen water droplets or any type of impurities
deposited at the upper surface of the water. In the absence
of such nuclei, water can remain in liquid phase at tem-
peratures well below its freezing point. For pure water, this
phenomenon is called supercooling. For impure water,
such as seawater, brackish, or even freshwater with low
salinity, it is known as constitutional or compositional
supercoiling, as will be clarified later in section 2.3.1.
Although by definition, pure water freezes at 0°C under
normal atmospheric pressure, it can be supercooled under
that pressure down to about −42°C. Considering the large
amount of dissolved impurities in seawater compared to
freshwater in lakes and rivers, the amount of supercooling
in seawater is probably a few hundredths to tenths of a
degree below the freezing point [
Weeks and Ackley,
1982].
Ice formation in freshwater entails the following pro-
cesses. As the water surface cools, the cooler layer at the
top becomes denser and therefore sinks, allowing warmer
water to rise. This vertical convection continues until the
surface temperature reaches the critical temperature of
maximum density, about 4.0°C. At this temperature the
convection essentially stops. Further cooling of the sur-
face layer makes it less dense and therefore remains at
the surface. The surface then responds faster to any
further drop in atmospheric temperature until it reaches
the freezing temperature of 0°C. At this point ice crystals
start to form around appropriate nuclei. It should also be
pointed out here that freshwater (depending on its purity
and the absence of nucleating agents) can be supercooled
well below that temperature if it is not mechanically dis-
turbed. The temperature of the water under the newly
formed ice away from the interface remains isothermal at
4.0°C. It gradually cools as a result of the heat exchange
at the ice‐water interface and the freezing continues. The
density of clear freshwater lake ice is slightly less than
about 917 kg/m
3
, or close to that of a single crystal of
2.1.2. Seawater Freezing Mechanism
As mentioned above, the water salinity is the controlling
factor that governs the temperature dependence of
both the freezing point and the density of the water.
Consequently, the freezing mechanism differs between
fresh, brackish, or seawater, though they all freeze when
the water surface is cooled down to or below its freezing
temperature. Additionally, freezing requires the presence