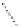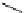Geology Reference
In-Depth Information
0
1000
1000
NaCI - H
2
O solution
(binary mix)
-5
500
500
Ice
-10
Ca
3
CO-6H
2
O
100
100
Na
2
SO
4
-10H
2
O
Usual salinity of
seawater 35‰
H
2
O
-15
50
50
NaCI-2H
2
O
MgCI
2
-8H
2
O
KCI
-20
CI
-
Eutectic point of NaCI = -22.8°C
H
2
O
10
MgCI
2
-12H
2
O
10
-25
Na
+
5
5
0
50
100
150
200
250
300
CI
-
SO
-
Salinity (‰)
Mg
++
+Ca
+
+K
+
+ rest
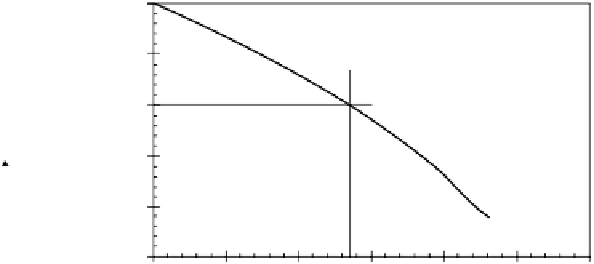
Figure 2.2
Phase diagram of binary mixture consisting of NaCl
and H
2
O.
1
1
0
-10
-20 -30
Temperature (°C)
-40
-50
-60
1001
Figure 2.1
Sea ice phase diagram [
Assur,
1958].
999.87 kg/m
3
999.97 kg/m
3
at 3.98°C
1000
From the viewpoint of materials science, natural sea
ice is a very complex material. In order to understand the
physics of sea ice, one must have an appreciation of the
so‐called sea ice phase diagram. A phase diagram illus-
trates the amounts and composition of the phases (ice,
brine, and precipitated solid salts) that exist at different
temperatures as a given volume of seawater freezes and
its temperature decreases. The reader must consult
Weeks
[2010] for a general understanding of this topic. Only a
very brief description will be given here. A comprehen-
sive phase diagram for “standard” sea ice was developed
by
Assur
[1958] and is presented in Figure 2.1. It shows
the weight ratio of each component: pure ice, salts, and
liquid brine at any temperature when the three phases are
in equilibrium. Temperatures for precipitation of differ-
ent salts are also shown. For all practical purposes, a
phase diagram for the binary mixture of sodium chloride
and water is sufficient to represent the salt contents in sea
ice. Only that part of the phase diagram is presented in
Figure 2.2. If brine exists in sea ice at −10°C, for example,
some water in the brine mixture has to freeze in order to
bring the mixture to its equilibrium concentration point,
i.e., to a salinity of 135‰ as shown in Figure 2.2. As the
temperature of the mixture decreases further, more water
molecules continue to solidify until the temperature
reaches −22.8°C, which is the eutectic temperature of
NaCl. At the eutectic temperature all sodium chloride
precipitates with no liquid left in the binary mixture.
999
998
Supercooled
region
997
996
995
-10
0
10
Te mperature, °C
20
30
Figure 2.3
Temperature dependence of density of pure water;
Note the maximum density at 4 °C.
solidification point in the temperature range of 4 °C-0 °C.
Lowering the temperature below 4 °C causes the density
not to increase, as expected, but to decrease as shown
in Figure 2.3. The maximum density (at 4 °C) is
999.972 kg/m
-3
. Another property of pure water, though
not unique to water, is the fact that it can be supercooled
far below the normal freezing point without solidifica-
tion. As the water is supercooled below the normal
freezing point, the density continues to decrease with
the decrease in temperature, as shown in the figure. This
behavior affects the freezing mechanism of pure water
as will be seen later in the next section.
Dissolved salts in water depress the temperature of
the maximum density, below that of pure water (about
4 °C) as well as the freezing point. For seawater, how-
ever, both the temperature of maximum density and the
freezing point decrease almost linearly as the water
salinity increases, as illustrated in Figure 2.4. At a criti-
cal salinity of 24.69‰, the two temperatures are equal
to −1.32°C. For salinities higher than the critical value,
the freezing temperature is higher than the temperature
of maximum density. At salinity of 35‰, the two tem-
peratures are −1.88 and −3.5°C, respectively.
2.1.1.3. Sea Ice Density
Another property of “pure” water, which is quite rele-
vant to ice formation and growth processes, is its density.
Much like any other material, water is subjected to ther-
mal expansion as its temperature rises or contraction
with the decrease in temperature. However, the behaviour
of pure water is very interesting very close to its