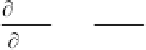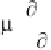Geology Reference
In-Depth Information
latent heat of fusion is very sensitive to temperature at
the near‐melting range. This is also where the sensitivity
to changes in salinity is pronounced. However, unlike the
specific heat, the latent heat decreases as temperature
or salinity increases. Results from equation (3.60) are
verified using data obtained from FY ice in McMurdo
Sound in the Antarctic over two winter seasons
Trodhal
et al.
[2000].
where
K
is called coulomb constant and
r
is the unit vector.
The constant can be described as
1
4
k
(3.62)
where
0
is the permittivity of free space.
A dielectric medium can be ideal or nonideal. Ideal
dielectrics possess no free charges to establish any conduc-
tion current, i.e., their conductivity is zero. They are homo-
geneous, isotropic, and lossless. Nonideal dielectrics possess
a very small number of free charges. Therefore, their electri-
cal conductivity is small but not zero. Permittivity is an
important property in both cases. Pure ice is almost an ideal
dielectric. Saline sea ice is a nonideal dielectric because
liquid brine contains free ions. Permittivity and conductivity
of dielectrics, measured in units of F/m, are usually com-
bined in a single parameter called the complex dielectric
constant or the complex permittivity , defined as
3.6. dielecTric properTies
The notion of the dielectric constant, which deter-
mines the microwave emission and scattering of sea ice,
is discussed here in some detail to furnish a background
for more discussions presented in Section 7.3. The
discussions can be useful for those who use the term
dielectric constant (also called complex permittivity)
and need to know more about its origin and how it is
related to other properties of sea ice, including the crys-
tallographic structure.
Unlike conductors that have free molecular charges
or insulators that have no such charges, a dielectric
substance contains a number of free molecules though
not appreciable. When an electrical field is applied to a
conductor the molecules move steadily, forming conduc-
tion current. The intensity of the current is determined
by the “conductivity” of the material. In response to
an applied electric field, molecules in a dielectric can
be displaced within molecular distances. The molecular
displacement can be established in either one of the
following two forms. If the molecules are originally
nonpolar, i.e., the center of charge of a molecule does
not coincide with its center of mass (as in the case of
the water molecules), then the displacement induces
polarization. If they are originally polar but randomly
oriented, then the displacement could induce a partial
alignment of molecules. In either case, the effect of an
externally applied electric field is to leave the interior
of the dielectric material uncharged and produce, instead,
a “bound” charge on surfaces normal to the field. Oscilla-
tions of these charges are manifested as an electric current,
called a displacement current, to flow through the die-
lectric. Strictly speaking, this current is determined by
the permittivity of the material. Permittivity of a dielec-
tric is the principle electrical property as conductivity is
for conductors.
At this point, it would be useful to briefly introduce
Coulomb's law and its relevance to permittivity. If
Q
1
and
Q
2
are two charges of the same polarity at a distance
r
from each other, then the force of repulsion
F
12
can be
written as
j
(3.63)
The real part is the permittivity and the imaginary part,
which is a function of the electrical conductivity, is called
the loss factor. Qualitatively speaking, permittivity deter-
mines the portion of energy that penetrates the material
(the rest will be scattered off the surface), while electrical
conductivity determines the portion of the energy that is
lost as heat or scattering inside the material. High permit-
tivity means less penetration of energy (hence more
reflection/scattering at the surface), while high loss means
more energy dissipation inside the material. The dielec-
tric constant of ideal dielectrics is a real number while
that of nonideal dielectrics is a complex number. The rea-
son for combining permittivity and loss into one complex
number and also for the negative sign in equation (3.63) is
clarified in the following mathematical formulation.
Wave propagation in an ideal (i.e., lossless) dielectric is
governed by the well‐known “wave” equation [
Lorrain
et al.,
1986]. For plane‐polarized waves propagating in
the
Z
direction, the electric field vibration in the
X
direc-
tion,
E
x
, is determined by the equation
2
2
E
Z
E
t
X
X
(3.64)
2
2
where is the permittivity and
μ
is the permeability of
the material (defined as the magnetic intensity caused by
a magnetic field of unit strength). Assuming harmonic
time dependence of the incident electric field
E
X
, with
magnitude
E
X
0
and frequency
ω
:
jt
EtREe
X
2
r
(3.61)
(3.65)
FKQQ r
12
/
e
X
0
12