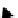Geology Reference
In-Depth Information
and vapor. In thermodynamics, the term latent heat is
the counterpart of the sensible heat, which, by definition,
causes change of temperature of the media as a result of
heat influence. For freshwater the latent heat of fusion at
0 °C is about 334 J/g, and the latent heat of vaporization
at 100 °C is about 2260 J/g (steam releases a great deal of
thermal energy when it condensates). Since sea ice con-
tains brine (liquid phase), its latent heat of fusion is less
than that of freshwater ice.
Malmgren
[1927] suggested
the following expression for the latent heat of fusion of
sea ice
L
si
as a function of the ratio of sea ice salinity to
brine salinity:
200
180
160
140
120
100
80
60
40
20
0
S
si
=10%
S
si
=8%
S
si
=6%
S
si
=10%
S
si
=2%
-10
-8
-6
-4
-2
0
Temperature (°C)
LL
S
S
b
Figure 3.25
Specific heat of sea ice as a function of ice tem-
perature and salinity, calculated from equation (3.58).
si
1
(3.59)
si
pi
no solid salt exists (the first major salt in sea ice, sodium
sulfate, precipitates at −8.2 °C):
where
L
pi
is latent heat of fusion of pure (freshwater)
ice. The higher the sea ice salinity (for constant brine
salinity) the less value of its latent heat. Phase change
from solid ice to liquid water is not the only process asso-
ciated with latent heat. That heat is required also to change
the phase of brine composition in order to maintain
the thermal equilibrium with the surrounding ice. This
process is not accompanied with change of temperature
of brine pockets or the surrounding sea ice.
Ono
[1967] developed the following expression to
calculate the latent heat of fusion of sea ice, which is
valid between 0 and −8 °C:
S
T
2 092 17210
2
C
.
.
si
(3.57)
si
2
si
where
C
si
is in J/g · K,
S
si
in ‰, and
T
in °C. A rationale
for using this equation is presented in
Ono
[1967] who
suggested also another equation based on an analytical
approach. It is also valid for ice temperatures between
0 and −8 °C and applies to FY ice. The equation has been
used in several studies [e.g.,
Pringle et al.,
2006]:
S
T
L
333 42113
.
.
T
0 114
.
S
18 04
.
si
C
2 113 0 0074
.
.
T
0 0034
.
S
si
si
si
si
si
si
si
S
T
0 00335
.
ST
000
.
376
T
si
2
(3.60)
5
84 10
.
ST
18 04
.
si
(3.58)
si
si
si
si
2
si
Here
L
si
is in J/g. For practical purposes, the last two
terms on the RHS can be neglected without compromis-
ing the accuracy. Calculations from this equation are
presented in Figure 3.26. Similar to the specific heat,
The units are the same as for equation (3.57). The first
term on the RHS represents the specific heat of pure ice.
Plots of calculations from this equation for different ice
temperatures and salinities are presented in Figure 3.25.
It can be seen that the specific heat capacity of sea ice
increases as temperature or salinity increases. The increase
with temperature is sharp above −3 °C. Below this temper-
ature the change in specific heat is marginal, and the values
from using different salinities stabilize around −4 J/g · °C
below −8 °C, regardless of the salinity (the plot is extended
to −10 °C although the equation is valid above −8 °C).
400
S
si
=2%
350
300
S
si
=4%
S
si
=6%
S
si
=8%
250
200
3.5.4. Latent Heat of Sea Ice
150
S
si
=10%
Latent heat of fusion is defined as the heat required for
changing the phase of a unit mass of material from the liq-
uid to solid state or vice versa at the same temperature. On
the other hand, the latent heat of vaporization or condensa-
tion is the heat required to change the phase between liquid
100
-10
-9
-8
-7
-6
-5
-4
-3
-2
-1
0
Temperature (°C)
Figure 3.26
Latent heat of sea ice as a function of ice tempera-
ture and salinity, calculated from equation (3.60).