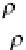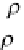Geology Reference
In-Depth Information
For very cold ice, when ice is largely solid with very
small brine volume, another equation based on conduc-
tion in a porous media has been suggested:
In other words, the brine volume is the key factor in deter-
mining the sea ice thermal conductivity.
Schwerdtfeger
[1963] developed a model for thermal
conductivity assuming sea ice as a compound bubbly
pure ice medium enclosing a number of vertical cylindri-
cal brine pockets (parallel to the heat flow direction). If
the thermal conductivity of sea ice is considered to be
equal to the summation of the conductivities of pure ice
and brine weighed by their cross‐sectional areas, then
K
si
is given by the following equation in terms of bulk sea ice
salinity
S
si
, which is an indicator of brine volume:
Va
K
K
aV
1011
.
b
b
pi
KK
(3.41)
si
pi
1011
.
b
where
K
K
b
a
1.
K
(3.42)
KKKK
S
pT
b
si
si
(3.45)
pi
si
pi
pi
b
si
b
According to equation (3.40) if brine volume in saline ice
is 10% (i.e.,
V
b
= 0.1) with brine conductivity
K
b
=
0.2 W/m · K,
K
si
drops to 2.23 from the value of 2.25 W/m · K
for pure ice. Air volume fraction also reduces
K
si
by the
ratio (1 −
V
a
)/(1 +
V
a
). This is a very small ratio. The effect
of solid salts on thermal conductivity of the sea ice mix-
ture is even smaller and often negligible.
Expressions for
K
pi
and
K
b
can be obtained following
the initial work of
Schwerdtfeger
[1963], which was com-
plemented by
Yen et al.
[1991]:
where
ρ
si
and
ρ
b
are, as usual, in kg/m
3
,
T
si
in °C,
S
si
in ‰,
and
p
is the slope of the phase boundary in the phase
diagram (1/
p
= −55 °C ). This equation is known as “bubbly‐
medium model” because it was derived based on the
assumption of a bubbly host material with brine inclusions.
Therefore, it is more valid for MY ice, especially the bub-
ble‐rich to layer of hummock ice. Another simple equa-
tion, which applies only to MY ice and is being used in
a few climate models, was developed by
Maykut and
Untersteiner
[1971] based on the one‐dimensional (1D)
thermodynamic sea ice model:
3
5
2
K
116191 86610
.
.
.
T
2 97 10
.
T
(3.43)
pi
si
si
S
T
2
K
0 4184 125003
.
.
.
T
0 00014
.
T
(3.44)
si
K
2030117
.
.
(3.46)
b
si
si
si
si
where the temperature is in °C and the conductivity is in
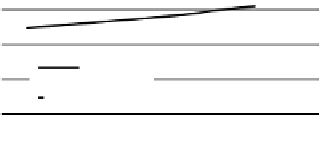
W/m · K. Plots of calculations from these two equations
are shown in Figure 3.19. Conductivity of pure ice increases
with temperature while conductivity of brine decreases
with temperature. These two opposite trends balance their
net effect on the conductivity of the sea ice mixture. This
implies that for the same brine volume the thermal con-
ductivity of sea ice is a weak function of ice temperature.
The Canadian Ice Service Community Ice-Ocean
Model (CIOM) uses this conductivity parameterization.
More recently,
Pringle et al.
[2007] proposed an alterna-
tive expression that is valid for bubbly‐rich ice (i.e., MY
ice) as well as brine pockets rich ice (i.e., FY ice):
S
T
si
pi
si
si
K
211011
.
.
T
009
.
(3.47)
si
si
1000
pi
si
3.0
The unit of each parameter is the same as used in the
previous equations, and
T
si
is in °C. This equation is the
best fit for data obtained from the bubbly‐medium model
and the “bubbly‐brine” effective medium model devel-
oped by
Pringle et al.
[2007]. The equation was verified
using in situ measurements of conductivity of sea ice in
the Arctic and Antarctic.
Thermal conductivity can be calculated from measure-
ments of temperature profile with thermistor or thermo-
couple arrays conducted in the field or in a laboratory
on either natural or artificial samples. Calculations
are complicated because of the possibility of perturb-
ing the local heat flow during measurements or possible
2.5
2.0
Pure ice
Brine
1. 5
1. 0
0.5
0.0
0
−
5
−
10
−
15
−
20
−
25
Temperature (
°
C)
Figure 3.19
Calculated thermal conductivity of pure ice and
brine for different temperatures. Note the divergence of the
values as temperature decreases.