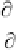Geology Reference
In-Depth Information
temperature and to melt ice crystals along the walls of
brine pockets. This is the essence of the model by
Bitz and
Lipscomb
[1999], which has been widely used in opera-
tional ice products.
Winton
[2000] presented a thermody-
namic model of sea ice suited for climate applications.
This is a three‐layer model that takes into consideration
the dependence of ice thermal properties on temperature
and salinity (simple models assume the ice sheet as a
single layer of fixed heat capacity). The upper ice layer is
given a variable heat capacity that ensures accurate
treatment of brine pockets. This model has been used to
produce ice products from NOAA (U.S. National Oceanic
and Atmospheric Administration) for many years. An
application of a one‐dimensional thermodynamic model
using different parameterizations of radiative fluxes is
presented in
Cheng
[2002]. A recent three‐dimensional
global model for sea ice dynamics and thermodynamics
has been developed by
Vancoppenolle et al.
[2009]. It is a
coupled ice‐ocean general circulation model (GCM)
where a simple representation of thermodynamics of
snow and ice is incorporated (e.g., snow is one layer of
constant physical properties). The performances of these
models rely on accurate estimates of thermal properties
of ice and snow and their variation with depth. These
estimates are discussed briefly in the following.
This causes the heat flows to be mainly in the vertical
direction. Obviously, ice grows in the direction of maxi-
mum heat flow. It is worth mentioning here that thermal
conductivity is a directional property. In a single ice crys-
tal, its value parallel to the
c
axis may be about 5% greater
than that along the normal to the
c
axis [
Fukusako,
1990].
Several authors determined the thermal conductivity of
YI and MY ice from in situ measurements of temperature
gradient. Other studies present models to calculate the
thermal conductivity. The estimated values from measure-
ments varied between different studies. This constitutes a
source of uncertainty in ice thermodynamic and climate
modeling. Even for freshwater ice the conductivity was
found to vary between 2.10 and 2.27 W/m · K with best
estimate to be around 2.25 W/m · K at −5 °C according to
Fukusako
[1990]. This can be attributed to different densi-
ties of defects that scatter the lattice vibrations responsible
for the conduction [
Yen,
1981].
Trodhal et al.
[2000, 2001]
discussed a set of Antarctic sea ice thermal conductivity
estimates from measurements conducted in McMurdo
Sound, Antarctica. They confirmed the temperature
dependence of the conductivity at subfreezing tempera-
ture range. Within the temperature range between −10 °C
and −30 °C the conductivity was nearly stable at
1.95 W/m · K. The authors provided evidence that change
of sea ice crystal orientation does not affect the heat flow
significantly unless the orientation affects the geometry
and distribution of the brine channels.
Pringle et al.
[2006]
presented thermal conductivity data from measurements
on FY ice and MY ice cores from the same area (McMurdo
Sound, Antarctic), which can be used as first approxima-
tion. They found that the thermal conductivity
K
si
of FY
ice within the top layer (0-100 mm) and a layer at depth
450-550 mm layers were 2.14 ± 0.11 and 2.09 ± 0.11 W/m · K;
respectively. For the significantly bubblier MY ice they
reported values around 1.88 ± 0.13 W/m · K. As mentioned
above, the liquid brine has considerably less thermal con-
ductivity than the solid ice. Therefore even a small increase
in brine volume at the ice surface in response to an increase
of atmospheric temperature above −5 °C will decrease the
thermal conductivity [
Weeks and Ackley,
1982].
The thermal conductivity of sea ice depends on tempera-
ture, salinity (including brine salinity), as well as the volume
fractions of brine and air. If air volume fraction is neglected,
the conductivity of the ice composition
K
si
becomes a func-
tion of the conductivities of the pure ice
K
pi
and brine
K
b
,
weighed by the volume fraction of the brine.
Anderson
[1958] presented this equation, which applies when the heat
is transferred vertically, i.e., during ice congelation:
3.5.1. Thermal Conductivity of Sea Ice
Thermal conductivity (
K
) is the ratio between the heat
flux (
F
) and the vertical temperature gradient:
FK
T
z
(3.39)
When water freezes, the thermal conductivity of ice
becomes much higher than that of water. For example,
the thermal conductivity of seawater is around 0.6 W/m · K
at 25 °C and salinity 35‰. At −20 °C and salinity 6‰
the thermal conductivity of sea ice is 2.10 W/m · K. In
general, thermal conductivity decreases with increasing
salinity and this applies to both water and ice. Freshwater
has slightly higher thermal conductivity (0.611 W/m · K)
compared to seawater and is commonly used by oceanog-
raphers to determine the salinity of seawater. Similarly,
freshwater ice has higher conductivity than sea ice. This is
because both inclusions in sea ice (brine and air) are poor
conductors. Thermal conductivity of air is 0.024 W/m · K,
i.e., two orders of magnitude lower than that of the ice.
The higher thermal conductivity of ice crystals with
respect to the brine explains the vertical growth of sea ice.
As mentioned in section 2.3.2, ice grows at the ice‐water
interface in a platelet pattern. That is because thermal
conductivity along the vertical ice platelets is much higher
than the conductivity across the brine between ice platelets.
KK V V
b
pi
101
.
0 1
.
(3.40)
si
b
b
where
K
b
is a function of temperature and salinity of the
brine as will be shown later.