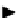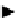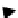Biomedical Engineering Reference
In-Depth Information
(a)
(b)
Normal droplet
volume
(c)
Normal droplet
volume
Abnormal
droplet volume
Figure 4.34
The parallel mixing and splitting test for a row of electrodes.
the preceding steps. As a trade-off, a more complicated test-result interpre-
tation scheme is required.
If all the tests in one row are executed without the detection of a malfunc-
tion, droplet volume should be almost the same. However, if a malfunction
occurs, volume variation is expected, as shown in Figure 4.34.
In Figure 4.34, the shaded droplet undergoes an unbalanced split during
the splitting test. Since all other droplets are split evenly, this malfunction
results in a pair of test droplets of abnormal volume, one bigger and the other
smaller. If the next step of the test yields no malfunction, the droplet volume
variation is propagated one electrode away. Therefore, we can easily detect
the malfunction by checking the test results.
The proposed test method achieves high efficiency. An implicit assump-
tion here is that adjacent electrodes are not defective. Such defects can be
detected by a separate structural test [36]. For an
N
×
N
array, only
N
2
+
N
manipulation steps are needed, while the test method in prior work [36]
requires 4
N
2
steps. Moreover, the method uses only one capacitive sensing
circuit, irrespective of the array size. This is in contrast to [36], which requires
N
2
capacitive sensing circuits for an
N
×
N
microfluidic array. The potential
reduction in production cost is therefore significant.
4.5.4 Application to Pin-Constrained Chip Design
In the discussion of the functional test method in Subsections 4.5.1-4.5.3, we
have assumed that the chip is controlled using the direct-addressing method.
In this subsection, we investigate the application of the functional test method
to pin-constrained biochips. Four different pin-constrained biochip proto-
types designed using the techniques mentioned in Chapter 3 are discussed.