Biomedical Engineering Reference
In-Depth Information
(a)
(c)
Fe@Fe
3
O
4
5 nm
5 nm
20 nm
MnFe
2
O
4
(b)
(d)
Fe@MnFe
2
O
4
10
3
Fe
3
O
4
CoFe
3
O
4
Fe@Fe
3
O
4
Fe@CoFe
2
O
4
mM
1.
0.4
0.2
0.1
0.05
0.025
10
2
Fe
3
O
4
10
1
Fe
3
O
4
CLIO
2.
10
0
6810 12 14 16
MION
3.
Diameter (nm)
10
-1
0
250
500
750
1000
M (emu·cm
-3
)
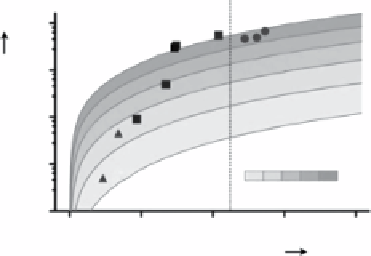
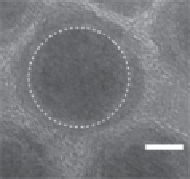
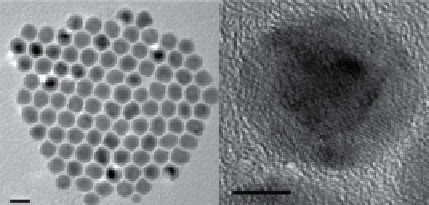
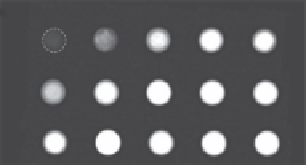
fIgure 2.7
(a) HRTEM image of an individual amorphous Fe/
fcc
-Fe
3
O
4
core-shell NPs
produced by thermal decomposition of Fe-oleate complex with Fe NPs. (b) Relaxivity
r
2
of
various ferrite NPs and amorphous Fe/ferrite core-shell NPs, indicating that the relaxivity of
NPs increases with size and magnetization and amorphous Fe/
fcc
-MnFe
2
O
4
core-shell NPs
show the highest
r
2
value. (c) TEM and HRTEM images of
bcc
-Fe/
fcc
-Fe
3
O
4
core-shell NPs.
(d)
T
2
-weighted images of the 16 nm
bcc
-Fe/
fcc
-Fe
3
O
4
NPs (1), amorphous Fe/
fcc
-Fe
3
O
4
NPs
(2), and Fe
3
O
4
NPs (3). (Reprinted with permission from Ref. [41]. © Wiley and Reprinted
with permission from Ref. [43]. © american chemical Society.)
Size-controllable monodisperse Fe NPs were synthesized by the thermal decom-
position of Fe(cO)
5
in octadecene solvent with oleylamine as the surfactant [24]. The
burst decomposition of Fe(cO)
5
at 180°c, yielding Fe atoms, cO gas and initiates
the nucleation and consequent growth [40]. The ratio of Fe(cO)
5
/oleylamine was
reported to be the critical factor to control the size of Fe NPs [24, 41]. The Fe NPs
produced in this method was amorphous and subject to air corrosion to form amor-
phous iron oxide on the surface. To protect the Fe NPs, the oxygen-transferring agent
(cH
3
)
3
NO was used to react with freshly made amorphous NPs at 250°c to generate
amorphous Fe/
fcc
-Fe
3
O
4
core-shell NPs, which had enhanced chemical and magnetic
stability [24]. another method was to thermally decompose the metal oleate complex
to form polycrystalline MFe
2
O
4
(M = Mn, Fe, co) on the surface of amorphous Fe
NPs, as labeled in Figure 2.7a [41, 42]. The produced 11 nm core/2.5 nm shell Fe/
MnFe
2
O
4
NPs, Fe/Fe
3
O
4
NPs, and Fe/coFe
2
O
4
NPs show the saturation magnetiza-
tions of 149, 142, and 133 emu·g
−1
(metal atoms), which were larger than one
of MnFe
2
O
4
of the same size (110 emu·g
−1
(metal)). These amorphous Fe/MFe
2
O
4
NPs were also made hydrophilic by dMSa. Measured at 0.47
T
field, the 16 nm
Search WWH ::

Custom Search