Biomedical Engineering Reference
In-Depth Information
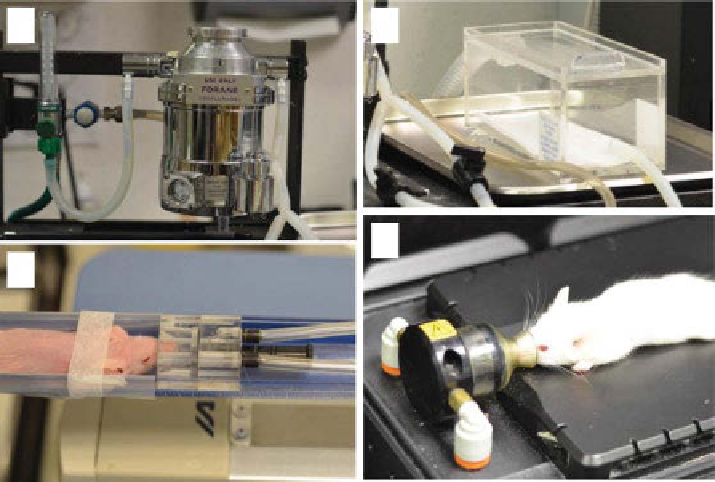
(a)
(b)
(d)
(c)
figure 16.2
Setup for inhalation anesthesia of laboratory animals for imaging. A precision
vaporizer (a) ensures delivery of desired concentration of anesthetic regardless of gas flow rate.
Animals are initially induced in anesthetic chamber (b) before being transferred to imaging bed
(c, d). Imaging platforms for nuclear imaging (c, nanoSPECT/CT, Bioscan) and optical imaging
(d, Pearl, lI-CoR Biosciences) utilize nose cones for direct delivery to mice with minimal
leaking to the environment.
used. Electrical heat plates can be used with caution as superficial burns can occur
if not carefully controlled. These plates may interfere with imaging in the case of
MRI, CT, and nuclear imaging. Heated-water circulation is a safer method and is
compatible with most imaging modalities. Heated, forced air is another option
when the animal is tightly contained or air can be constantly blown over the sub-
ject. Many commercial preclinical imaging systems feature methods for maintain-
ing normothermic conditions.
16.3.5
Positioning and motion
Adequate maintenance of position and reduction of motion are important factors
in acquiring reproducible and comparable imaging datasets. This is especially true
for planar imaging modalities including X-ray, scintigraphy, fluorescence reflec-
tance imaging (FRI), and bioluminescence imaging (BlI). While three-dimensional
(3D) tomographic imaging modalities are not as dependent on position, reliable
determination of anatomical features is best when animals are positioned
correctly.
Imaging of the skull and brain is generally accomplished by holding of the head
in a modified stereotactic frame. This accomplishes several purposes including
Search WWH ::

Custom Search