Biomedical Engineering Reference
In-Depth Information
(a)
(b)
Surfactant exchange
+
A
+
B
Surfactant addition
Metal precursor
Surfactant
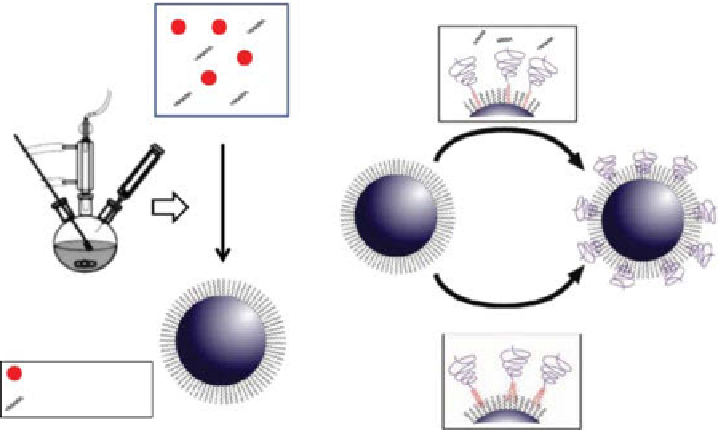
fIgure 2.3
Schematic illustrations of (a) a typical organic solution phase synthesis of
IOMNPs (b) two strategies used for converting hydrophobic NPs into hydrophilic ones.
(Reprinted with permission from Ref. [10]. © Wiley.)
with high boiling point is also necessary to provide the desired decomposition
condition. To control NP nucleation and growth, the iron precursor is either “hot
injected” into the preheated solvent or premixed with other surfactants in the organic
solvent followed by heating. The nucleation happens when the rapid decomposition
of iron precursors at high temperature yields an over-supersaturated monomer solu-
tion in a short interval, which is called “burst nucleation.” This burst nucleation con-
sumes most monomers and prevents any further nucleation [21, 22]. The nuclei then
grow up to monodisperse NPs at the same rate, depleting the rest of monomers.
another avenue to IOMNPs is
via
oxidation of the metallic Fe NPs, which are
preformed by the thermal decomposition of iron pentacarbonyl (Fe(cO)
5
) [23, 24].
The Fe NPs can also be oxidized in a more controlled manner, giving core-shell
Fe/iron oxide NPs, iron oxide NPs, or hollow iron oxide NPs [25].
In organic solution phase synthesis, the presence of a lipid-type surfactant is critical
to control the NP growth into different sizes and shapes and to stabilize the produced
MNPs [26]. The bipolar surfactant always has a polar group binding with the surface
atoms and a hydrocarbon chain to provide steric protection of the NPs. Oleic acid and
oleylamine are the common surfactant combination used in NP synthesis and stabili-
zation. These surfactant-capped MNPs can form very stable dispersion in a nonpolar
solvent. However, to make them suitable for biological applications, these MNPs need
to be further modified. as shown in Figure 2.3b, one well-established method is to do
ligand exchange, replacing the initial hydrophobic surfactant with a hydrophilic cap-
ping agent that has a stronger binding power to the NP surface [10]. as a typical
example, 2, 3-dimercaptosuccinic acid (dMSa) can replace the hydrophobic capping
Search WWH ::

Custom Search