Biomedical Engineering Reference
In-Depth Information
-
O
O
C
N
O
O
N
C
Gd
C
O
N
C
H
O
-
O
O
C
-
HN
figure 1.8
amphipathic gd complex with DTPa featuring hydrophobic tails. DTPa
anhydride was reacted with stearyl amines and integrated into the lamellar phase of liposome
particles. The
T
1
in the liver increased by 180%. (Based on ref. [36].)
shortening of the
T
2
or
T
2
* relaxation times. a group led by J. leigh Jr., a radiology
scientist at the University of Pennsylvania, introduced ferromagnetic, albumin-coated
magnetite particles of 1-50 nm in diameter [39]. These nanoparticles, prepared from
ferric and ferrous chlorides, were covered with cross-linked albumin to decrease
potential metal toxicity and showed significant reduction of both
T
1
and
T
2
at
relatively low particle concentration in rats. at the same time, P. lauterbur's group
from the State University of New York, Stony Brook, reported enhanced imaging of
the abdomen in dogs using 50 nm magnetite nanoparticles [40]. (The contribution
of P. lauterbur in MrI was acknowledged with a Nobel Prize in 2003.) Both leigh's
and lauterbur's groups predicted the success of ferromagnetic nanoparticles in future
biomedical research and clinical diagnosis.
In the upcoming years, the potential of many types and sizes of superpara-
magnetic particles (SMP), micrometer-sized paramagnetic iron oxide (MPIO,
micron size), superparamagnetic iron oxide (SPIO, submicron size), and ultrasmall
superparamagnetic iron oxide (USPIO, <50 nm) has been evaluated in many pre-
clinical and clinical trials. The acceptance of these particles in research and
clinical applications was mainly due to the following advantages: (i) a strong
change in the signal per iron atom, in particular on
T
2
-weighted images (given
thousands of iron atoms in the nanoparticle, the strength of the signal overcomes
the typical low contrast agent sensitivity of MrI); (ii) the biocompatibility of
relatively low toxicity iron—free iron can be metabolized via normal biochemical
pathways; and (iii) coating and surface modifications, allowing the active targeting
of the nanoparticles [41]. Many of the SMP nanoparticles have been commercial-
ized and approved by healthcare systems in europe and asia under the trade names
of endorem®, guerbet Sa, or ferridex®, Berlex laboratories, Inc. By the turn
of the twenty-first century, contrast agents were used in nearly 25% of all MrI
procedures.












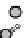















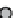















































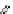























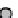






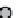























Search WWH ::

Custom Search