Biomedical Engineering Reference
In-Depth Information
O
-
O
-
O
O
O
O
H
N
H
N
O
O
H
N
H
N
O
O
x
y
NN
N
N
Gd
3+
L
-Cysteine
x
y
+
O
-
O
-
Gd
3+
O
O
N
N
NN
-
O
O
HN
O
S
-
O
O
O
HN
NH
NH
-
O
SH
-
O
HS
S
O
O
Poly(
L
-glutamic acid)-cystamine-[Gd(III)-DO3A]
chaRt 8.4
example of a biodegradable polymer and its fragments after incubation with
l-cysteine.
and the potential toxicity due to the released Gd
3+
. This slow excretion rate issue is
why efforts are being made to develop polymers that are degraded
in vivo
into smaller
parts for faster elimination.
Most of the biodegradable polymers for MRI were developed in Lu's group [66-68].
They are derivatives of the polymers described previously with disulfide bonds in their
structure allowing cleavage into smaller residues by endogenous thiols such as cysteine
and glutathione (Chart 8.4). For instance, the lateral chain of poly-(l-glutamic acid)
was conjugated to dO3A with a cystamine spacer and labeled with Gd
3+
. The resulting
polymer exhibited enhanced blood pool contrast and fast excretion of the Gd
3+
-dO3A
chelate through renal filtration [66]. similarly, peGylated Gd
3+
-dTpA copolymerized
with l-cystine exhibited good blood pool agent characteristics with fast elimination [67].
However, a degradation that would occur too fast is not desirable, and it was
demonstrated that the rate of disulfide bond cleavage could be controlled by intro-
ducing steric hindrance around it to limit accessibility by endogenous thiols [68].
Alternatively to disulfide bond investigations, Wen
et al
. studied the influence of the
Gd
3+
-dTpA linkage on the degradability of poly-(l-glutamic acid). A higher degra-
dation rate was observed with a benzyl linker as compared to a hexyl in the presence
of cathepsin B, a lysosomal enzyme [69]. Another interesting approach is the use of
protein polymers proposed by Karfeld-sulzer
et al
. that consist of repeated amino
acid units produced by genetic engineering conjugated to Gd
3+
-dTpA on the amino
groups of the lysines [70]. size of the polymer and lysine spacing could be controlled
to modulate the relaxivity (up to 461 mM
−1
s
−1
at 60 MHz, 37°C).
In vitro
studies
indicated degradation by plasmin, a naturally occurring enzyme that cleaves on the
carboxyl side of lysine residues.
Although not strictly belonging to the linear polymers, carbohydrate derivatives
do deserve mention in this section. With molecular weights up to 150 kda, carbohy-
drates such as dextran, starch, and inulin have been investigated, and, as for the linear
polymers described previously, high relaxivities and long circulation times were
obtained, making them good blood pool agents for visualization of various lesions
and diseases. Carbohydrates have the advantage of being naturally present in the
body in various shapes. That is why their Gd
3+
derivatives exhibit low immunogenicity
and low toxicity. Furthermore, they are readily metabolized by various processes
allowing their excretion. A good example is the use of hyaluronan proposed recently
by Zhu and Artemov. Hyaluronan is a linear glycosaminoglycan approved by the
FdA for various medical applications. It is degraded by hyaluronidase in lymph
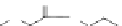





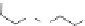






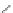
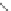
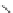


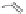






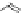



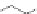









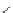
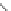
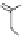




Search WWH ::

Custom Search