Biomedical Engineering Reference
In-Depth Information
taBle 8.1
description of approved Gadolinium-based contrast agents
plasma relaxivity
(mM
−1
s
−1
) at
1.5 T, 37°C
a
Formal
charge
Chemical name
Trade name
use
Gd-dTpA
Magnevist
4.1
2
−
eCF
Gd-dTpA-BMA
Omniscan
4.3
0
eCF
Gd-dTpA-BMeA
OptiMARK
4.7
0
eCF
Gd-dOTA
dotarem
3.6
1
−
eCF
Gd-HpdO3A
proHance
4.1
0
eCF
Gd-dO3A-butrol
Gadovist
5.2
0
eCF
Gd-eOB-dTpA
eovist (united states),
primovist (eu)
6.9
2
−
eCF + liver
Gd-BOpTA
MultiHance
6.3
2
−
eCF + liver
Ms-325
(gadofosveset)
Ablavar (united states),
Vasovist (eu)
27.7
3
−
Blood pool
a
Values from Aime and Caravan [18].
(c1)
(a)
(c2)
(b1)
(b2)
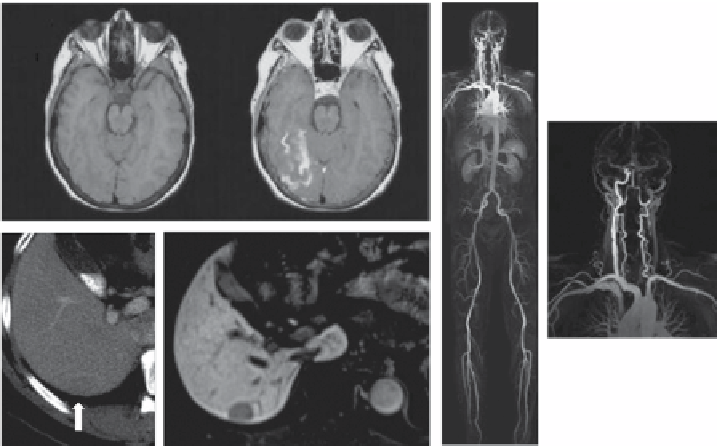
FIGuRe 8.2
examples of contrasted pictures obtained with the three classes of approved
Gd-based contrast agents (CAs). (a) MRI of the brain of a patient after an acute stroke event. The left
picture is a
T
1
-weighted acquisition without CA. The right picture shows a cerebral hemorrhage after
administration of Gd-dTpA (Reprinted with permission from Ref. [36]. © Wolters Kluwer Health).
(b1) Visualization of a grade I hepatocellular carcinoma in the liver without CA. (b2) 20 min after
administration of Gd-eOB-dTpA, the high contrast allows a better diagnosis of the tumor (Reprinted
with permission from Ref. [37]. © Hindawi publishing Corporation). (c1) Whole-body angiography
after administration of Ms-325 produces strong contrast, particularly in the supra-aortic/thoracic
region. (c2) A zoom from the same picture into the neck region shows the occlusion of the left carotid
artery (Reprinted with permission from Ref. [38]. © Wolters Kluwer Health).












Search WWH ::

Custom Search