Biomedical Engineering Reference
In-Depth Information
Since there is a huge variability in nanoparticle composition (organic vs. inorganic/
metal based), standard radiolabeling and purification steps are not applicable across
the board. However, the end-term characterization goals are the same. one of the
critical challenges with determining the specific activity of radiolabeled nanoparti-
cles is that occasionally determining the “exact mass” of the column eluted/purified
radiolabeled nanoparticle solution is not exactly known, primarily because the molar
absorptivity of the nanoparticles at low concentrations is not enough for ultraviolet
(UV) detection. Researchers typically approach this challenge by making the “theo-
retical mass calculation” based on experience and calibration curves constructed by
using known standards.
Standard steps in the characterization of organic nanoparticle radiolabeled with a
radionuclide involve the use of chromatography techniques [3]. a radioactive/UV
chromatogram can be utilized in the following fashion:
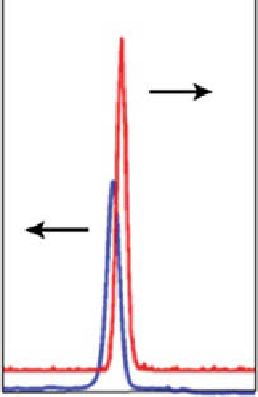
1. a fast protein liquid chromatography (fPlC) or radio thin-layer chromatog-
raphy (R-TlC) chromatogram of the mixture of radionuclide and nanoparticle
before purification (fig. 6.14). Ideally, a radioactive peak and the corresponding
UV peak (fPlC) will appear for both nanoparticle radioactive conjugate and
“free” unconjugated radioactive peak. This scan will determine the radiolabel-
ing efficiency.
2. The chromatogram should be recollected after purification (such as passage of
the reaction mixture over a Zeba desalting column). This scan will determine
the purity of the radiolabeled nanoparticle solution.
Retention time (min)
figure 6.14
a schematic of radio and UV spectrum of a radiolabeled nanoparticle conjugate.
Search WWH ::

Custom Search