Chemistry Reference
In-Depth Information
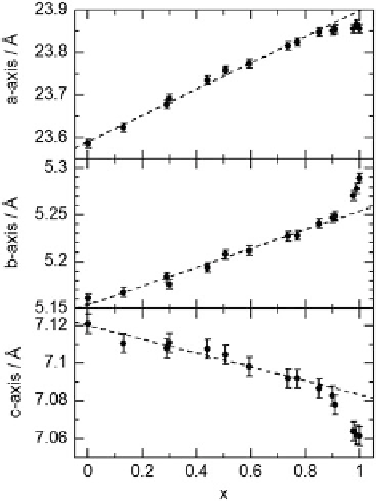
Fig. 4.11 Unit cell
parameters of
[Ni
1
x
Pd
x
(chxn)
2
Br]Br
2
as a
function of
x
.
Dashed lines
show fitted data
chains are connected to each other via hydrogen bonds
ð
N
H
Br
H
N
Þ
along the
c
axis. Therefore, in order to maintain the hydrogen bonding, the length of
the
c
axis decreased with an increase in
x
instead.
The unit cell length in each direction obeys a linear relationship when
x <
0.9,
whereas it deviates from the line when
x >
0.9, especially in the case of the
b
and
c
axes. The
x
dependence of the unit cell length of the
b
axis (//1D chain) could be
influenced by the electronic state of the compound. When the compound is in the
MH state, the unit cell length of the
b
axis should be simply twice the linear
summation of the Ni(III)-Br and Pd(III)-Br bond lengths as follows:
b ¼
2
f
ð
1
xÞdð
Ni
ð
III
Þ
Br
Þþxdð
Pd
ð
III
Þ
Br
Þ
g;
where
dð
Ni(III)
Br
Þ
and
dð
Pd(III)
Br
Þ
indicate Ni(III)-Br and Pd(III)-Br
bond lengths, respectively.
On the other hand, in the CDW state, the oxidation states of Ni and Pd ions are
supposed to be Ni(II) and Pd(IV), respectively, because Ni ion cannot have the
oxidation state of Ni(IV) due to the higher oxidation potential from Ni(III) to Ni
(IV) than that from Br
to Br
0
. Therefore,
b
axis should be expressed as follows:
b ¼
f
ð
xÞdð
ð
Þ
Þþðx
:
Þdð
ð
Þ
Þþ
:
5
dð
ð
Þ
Þ
g;
2
1
Ni
II
Br
0
5
Pd
II
Br
0
Pd
IV
Br
where
dð
Ni(II)
Br
Þ
,
dð
Pd(II)
Br
Þ
, and
dð
Pd(II)
Br
Þ
indicate Ni(II)-Br, Pd
(II)-Br, and Pd(IV)-Br bond lengths, respectively.
Search WWH ::

Custom Search