Chemistry Reference
In-Depth Information
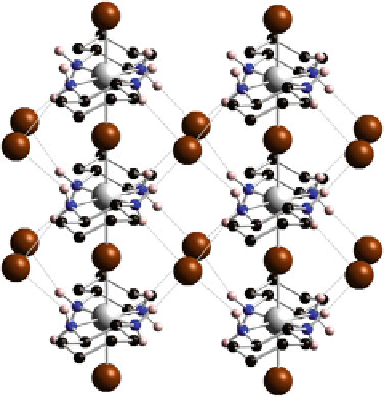
Fig. 3.1 Crystal structure of
[Ni(chxn)
2
Br]Br
2
.
Gray
: Ni,
Brown
: Br,
Black
:C,
Blue
:N,
Pink
:H
between the Ni and the Pd and Pt ions. In the Ni complexes, bridging Br
ions are
located at the midpoints between neighboring Ni ions, whereas they are displaced
from the midpoint in Pd or Pt compounds and are disordered [
4
]. This indicates that
the Ni ions are in a trivalent Ni
III
Mott-Hubbard (MH) state. There are hydrogen
bonds between the amino protons and counteranions, forming a 2D hydrogen-bond-
network. Ni-Ni distances along the 1D chain are 5.161(2)
˚
.
3.3 Magnetic Properties
Figure
3.2
shows the temperature dependence of the magnetic susceptibility of
[Ni(chxn)
2
Br]Br
2
measured as a single crystal [
3
]. Curie-like behavior was observed
at low temperatures, whereas nearly no temperature dependence was observed at
higher temperatures. The small amount of spin concentration showing Curie-like
behavior (
0.4 %) was attributed to some paramagnetic impurities or spins at the
chain end. The weak temperature dependence of the susceptibility at high tempera-
ture region was interpreted to be due to an
S ¼
1/2 Heisenberg chain with strong
antiferromagnetic (AF) interactions. Such temperature dependence of susceptibilities
can be understood via the Bonner-Fisher theory [
5
]. Recently, Eggert, Affleck, and
Takahashi (EAT) have proposed a theory that explains more accurately the
susceptibilities, which slightly differ
from Bonner-Fisher behavior at
low
temperatures [
6
]. The result of EAT theory for
T <
0.2
J
/
k
B
is given by the following
equation:
Ng
2
m
B
2
2
Jp
1
wðTÞ¼
þ
;
1
(3.1)
2
2ln
ð
15
:
4
J=k
B
TÞ

Search WWH ::

Custom Search