Chemistry Reference
In-Depth Information
10
2
13
10
0
2
14
3
5
10
-2
4
6
10
-4
7
10
-6
1.0
1.5
2.0
2.5
3.0
E
CT
(eV)
Fig. 2.8 Normalized integrated intensities of the luminescence as a function of
E
CT
in the MX
chain compounds. The material for each number is listed in Table
2.1
. Luminescence is measured
at 2 K with the excitation photon energy of 2.4 eV. Data of the Pt compounds are represented by
circles
. Data of the heterometal compounds with M
¼
Pt and Pd are represented by
diamonds
.
Open
and
filled marks
indicate the compounds with Y
¼
ClO
4
and Y
¼
halogen, respectively.
(Reprinted figure with permission from [
5
])
a ¼ kl
) of [Pt
(en)
2
][Pt(en)
2
Br
2
](ClO
4
)
4
-II single crystal with the excitation of 3.2 eV at 77 K.
Here,
k
and
l
are the absorption coefficient and the sample thickness, respectively.
Polarization of both the irradiation light (
E
ex
) and the transmission light (
E
)is
parallel to the chain axis
b
. In Fig.
2.9a
, the polarized absorption spectrum for
E
//
b
was also shown by the broken line. The arrow indicates the optical gap energy
E
CT
.
A midgap absorption band labeled as
a
at 1.55 eV and a weak shoulder structure
labeled as
b
at 1.79 eV were observed in as-grown samples. These absorption bands
were enhanced by light irradiations. In addition, a weak PA band labeled as
g
was
observed at 0.7 eV. To discriminate the observed PA bands, time characteristics of
three structures were investigated. The results showed that bands
a
and
b
exhibited
the same decay characteristics, while band
g
decays in a different manner compared
with
a
and
b
. These results suggest that optically excited states include two
different photoproducts associated with
a
,
b
, and with the lower energy band
g
.
The absorption and PA spectra in the heterometal compound, Pt-Br-Pd, were
shown in Fig.
2.9b
. They were considerably different from the spectra in Pt-Br-Pt-II.
In the absorption spectrum below the optical gap energy
E
CT
, there were no promi-
nent structures. By the 3.2 eV excitation, two PA bands appeared, which were labeled
as a
1
and a
2
. a
1
and a
2
were found to show the same decay characteristics, suggesting
that they are related to the same excited species.
To deduce the generation process of the photoproducts, excitation profiles of PA
bands (
a
and
g
in Pt-Br-Pt-II, and a
1
in Pt-Br-Pd) were measured. The results are
shown in Fig.
2.10
by circles for band
a
, triangles for band
g
in Pt-Br-Pt-II, and the
Figure
2.9a
shows the PA spectrum
Da
(photoinduced change of








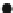





























Search WWH ::

Custom Search