Chemistry Reference
In-Depth Information
AV
X
M
2.5+
M
2.5+
X
2.5+
M
2.5+
X
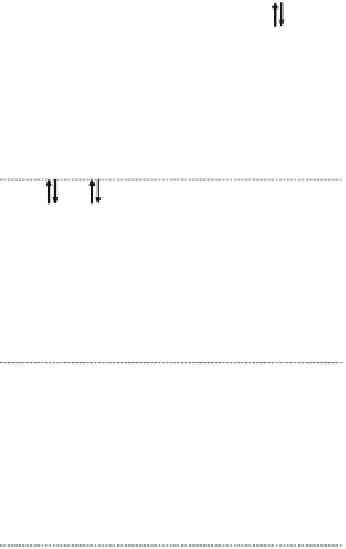
CDW
X
M
2+
M
2+
X
3+
M
3+
X
CP
X
M
2+
M
3+
X
M
2+
M
3+
X
ACP
X
M
2+
M
3+
X
3+
M
2+
X
Fig. 12.1 Schematic electronic and lattice structures of MMX compounds
n
H
2
O with X
Cl and Br are known to be in the CDW phase [
1
-
4
]. Meanwhile,
the compounds with X
¼
I have been studied extensively and show different
electronic phases [
5
-
7
]. The mechanism of the variation of the electronic phases
is clarified here. (2) In Pt
2
(RCS
2
)
4
I, the ligands surrounding the binuclear MM unit
are RCS
2
with R
¼
¼
CH
3
,C
2
H
5
,
n
-C
4
H
9
, etc
.
Among them, the compound with
R
CH
3
CS
2
) has been studied for many years [
8
]
and has been found to show the AV phase with “metallic” conductivity above room
temperature [
9
,
10
]. The compound with R
¼
CH
3
(the ligand is then dta
¼
¼ n
-C
4
H
9
clearly shows the ACP phase
[
11
]. The difference between the pop and dta systems is discussed here from the
theoretical viewpoint.
The more degrees of freedom, compared with those in the MX compounds, are
responsible for the greater variety of electronic phases in the MMX compounds.
Furthermore, the smaller charge gap enhances the controllability of relative stabil-
ity among the four phases above. Indeed, electrons are more delocalized than in the
MX compounds. Among the theoretical studies into the origins of the charge and





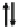



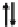
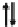




































Search WWH ::

Custom Search