Chemistry Reference
In-Depth Information
a
b
CP state
Pt
2
+
Pt
3+
Pt
2
+
Pt
3
+
I
I
I
p
z
d
z
2
t
MM
,V
MM
V
2
t
MXM
, V
MXM
light, pressure
CDW state
Pt
2
+
Pt
2
+
Pt
3
+
Pt
3
+
[(C
2
H
5
)
2
NH
2
]
4
[Pt
2
(pop)
4
I]
I
I
I
c
Pt
I
P
C
a
b
O
N
Fig. 11.2 (a) Crystal structure of [(C
2
H
5
)
2
NH
2
]
4
[Pt
2
(pop)
4
I]. H atoms have been omitted for
clarity. (b) Schematic electronic structures of CDW and CP.
t
MM
and
t
MXM
denote the electron
transfer energies.
V
MM
,
V
MXM
, and
V
2
denote the Coulomb interactions
ions greatly alters the distance
d
(Pt-I-Pt) between two Pt ions bridged by the I ion.
Such control of
d
(Pt-I-Pt) makes it possible to control the various electronic
parameters such as intersite
e
-
e
interaction and
e
-
l
interaction and to realize the
paramagnetic CP state as well as the diamagnetic CDW state.
Figure
11.2a
represents the structure of a typical PtPtI chain,
[(C
2
H
5
)
2
NH
2
]
4
[Pt
2
(pop)
4
I]. Two Pt ions are linked by four pop molecules, forming
a binuclear Pt
2
(pop)
4
unit. The two neighboring Pt
2
(pop)
4
units are bridged by I and
the PtPtI-chain structure is formed along the
c
axis. The iodine ion deviates from the
midpoints between the two neighboring Pt ions, indicating that this compound is in
CDW state or CP state, as illustrated in Fig.
11.2b
. Figure
11.2a
shows the two
possible positions of I as the displacements of I are not three-dimensionally
ordered. X-ray structural analysis was unable to resolve whether the ground state
is CDW state or CP state. CP state is composed of Pt
2+
-Pt
3+
units, and the Pt
3+
ions
have spin (
S ¼
1/2), forming a 1D antiferromagnetic spin chain. CDW state on the
other hand is composed of Pt
2+
-Pt
2+
and Pt
3+
-Pt
3+
units and is diamagnetic since
the two neighboring Pt
3+
ions form singlet states. The ground states of the PtPtI
chains can then be discussed keeping these characteristics of the two charge
ordering states in mind.
Figure
11.3a
shows the optical conductivity spectra of [H
3
N(C
6
H
12
)
NH
3
]
2
[Pt
2
(pop)
4
I] and [(C
2
H
5
)
2
NH
2
]
4
[Pt
2
(pop)
4
I] as typical examples. The optical
gap energies
E
gap
(1.0 and 2.4 eV) of the two compounds differ considerably. The
Raman-scattering spectra are shown in the inset to provide information concerning
the valence of M [
3
-
5
]. Strong bands at 80-100 cm
1
are attributed to the Pt-Pt































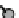

































































































































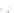










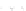










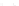

















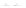













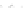



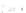















































Search WWH ::

Custom Search