Chemistry Reference
In-Depth Information
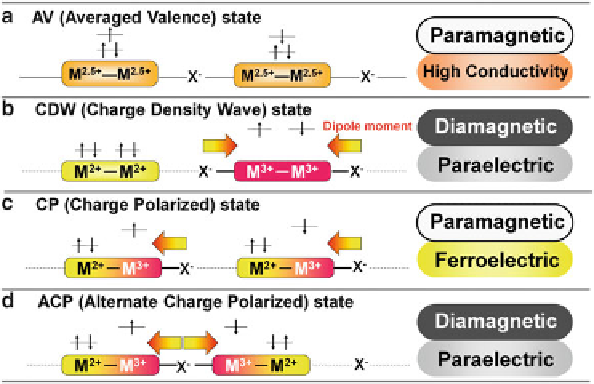
Fig. 10.1 Electronic state and expected physical properties of MMX chains
In contrast to the stability of Pt(II)-Pt(II) and Pt(III)-Pt(III) complexes, mixed
valence Pt(II)-Pt(III) complex is quite unstable in the solution because of the
disproportionation to Pt(II)-Pt(II) and Pt(III)-Pt(III) complexes. Very recently,
one Pt(II)-Pt(III) complex was reported in CH
2
Cl
2
, the weakly coordinating sol-
vent, and its structure was determined as the discrete Pt(II)-Pt(III) complex,
(PPN)
3
[Pt
2
(pop)
4
(NO)]
PPh
3
]
+
), by the
single-crystal X-ray structural analysis [
15
]. However, the overwhelming number
of the Pt(II)-Pt(III) complexes have been reported as the infinite linear-chain
complexes, which are in attractive one-dimensional (1D) electron system. This
intriguing study of mixed valence chain complexes was triggered by the discovery
of K
4
[Pt
2
(pop)
4
Br]
(PPN
+
2Et
2
O
CH
2
Cl
2
¼
[Ph
3
P
¼
N
¼
3H
2
O in 1983 [
16
].
10.1.2
Introduction to pop-Type MMX Chains
The linear-chain complexes based on pyrophosphito-bridged diplatinum complex are
called as pop-type “quasi-1D halogen-bridged dinuclear metal complexes (MMX
chains).” Although 1D electron system of quasi-1D halogen-bridged mononuclear
metal complexes (MX chains) originates two electronic states bringing about unique
physical properties as shown in Part I, MMX chains have attracted intense interest,
because their higher degrees of freedom of the electrons provide the competition and/
or cooperation among more diverse energetic factors, which causes a larger variety of
electronic states and smaller energy gaps among them. Based on the theoretical
MMX chains can be classified into four states as shown in Fig.
10.1
.
These electronic states are strongly correlated to the position of the bridging
halide ion. Except for AV state, the bridging halide ion is close to M
3+
ion. A formal
Search WWH ::

Custom Search