Chemistry Reference
In-Depth Information
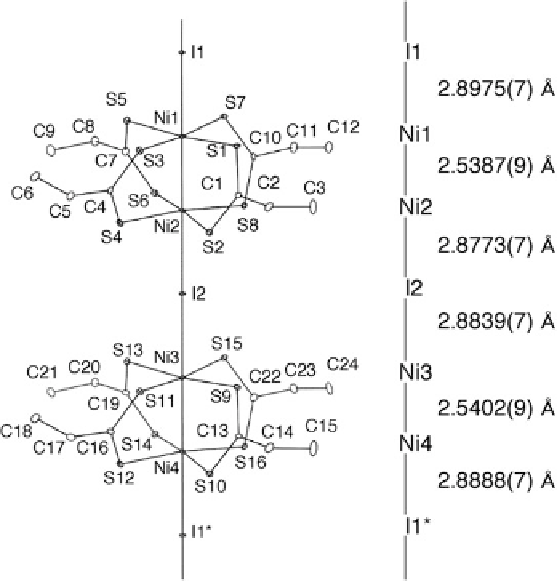
Fig. 9.39 1D chain structure of [Ni
2
(EtCS
2
)
4
I]
1
(8) at 26 K with an atomic numbering scheme
and relevant interatomic distances (thermal ellipsoid set at the 50 % probability level) [
38
]
here based on the relative arrangement of 1D chains in the crystal, which minimizes
the Coulomb repulsion between them. As shown in Fig.
9.40
, there are two types for
the arrangements of 1D chains [
38
].
Adjacent 1D chains in arrangement type (a) shift about a half period from each
other, whereas the 1D chains in arrangement type (b) are aligned with the same
phase. Here I discuss the twofold periodic valence ordering which minimizes the
Coulomb repulsion between the 1D chains. The type (b) arrangement is capable of
taking both valence-ordered ACP and CDW states. [M
2
(MeCS
2
)
4
I]
1
(M
¼
Ni (7),
Pt (1)) have relatively short interchain S
S contacts in the type (a) arrangement,
and these compounds are considered to have two-dimensional interactions [
28
,
37
].
When a 1D chain takes the ACP state in type (a), adjacent 1D chains should take the
CDW state to minimize the Coulomb repulsion. As a result, it is presumed that these
compounds would have difficulty adopting the superstructure associated with the
twofold periodic valence ordering, and consequently, [Ni
2
(MeCS
2
)
4
I]
1
(7) would
be difficult to show the spin-Peierls transition associated with the ACP state. On the
other hand, it is possible for [Ni
2
(RCS
2
)
4
I]
1
(R
Et (8),
n
-Pr (9)) to adopt twofold
periodic valence ordering, since the one-dimensionality of these compounds
¼
Search WWH ::

Custom Search