Chemistry Reference
In-Depth Information
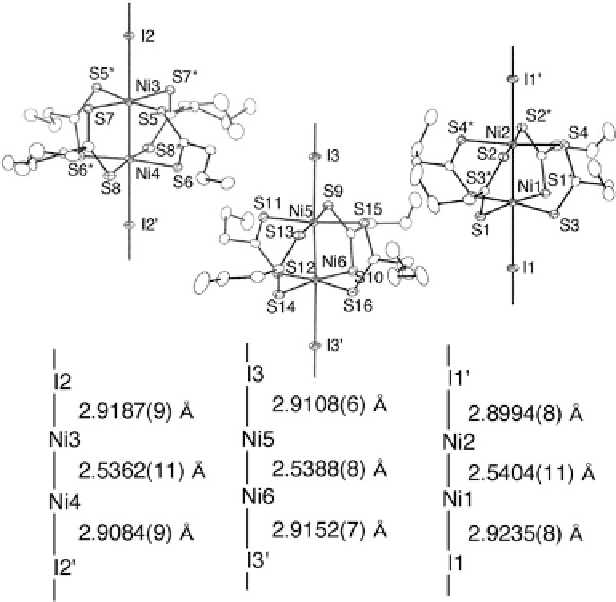
Fig. 9.33 1D chain structures of [Ni
2
(
n
-PrCS
2
)
4
I]
1
(9) in the LT phase at 140 K with an atomic
numbering scheme and relevant interatomic distances (thermal ellipsoid set at the 50 % probability
level) [
38
]
Ni
2+
-Ni
3+
mixed-valence state, the intensity of the Ni
3+
2p
3/2,1/2
doublet is very weak
compared to that of Ni
2+
. This would be due to the reduction of Ni
3+
to Ni
2+
by X-ray
irradiation similar to the observation made for [Pt
2
(EtCS
2
)
4
I]
1
(2)[
32
].
9.3.4 Electronic State
Electronic absorption spectra of [Ni
2
(RCS
2
)
4
I]
1
(R
Et (8),
n
-Pr (9),
n
-Bu (10))
are shown in Fig.
9.35
, together with that of [Ni
2
(EtCS
2
)
4
][
38
]. Spectral data of
7-10 are summarized in Table
9.6
[
38
,
82
]. The dominant feature of the absorption
spectra of 7-10 is an intense sharp band centered at 5,200-5,600 cm
1
, which is absent
from the spectra of [Ni
2
(RCS
2
)
4
](R
¼
Me, Et,
n
-Pr,
n
-Bu). To elucidate the electronic
structure of the [Ni
2
(RCS
2
)
4
I]
1
, UB3LYP method has been applied to the model
structure of [Ni
2
(MeCS
2
)
4
I]
1
(7)[
38
]. HOMO and LUMO are composed of Ni d
z
2
¼
s
combination. Those HOMO and LUMO are assigned to a lower Hubbard
Ni d
z
2
(LH) d
s
* and an upper Hubbard (UH) d
s
* orbitals, that split by an on-site Coulomb
Search WWH ::

Custom Search