Chemistry Reference
In-Depth Information
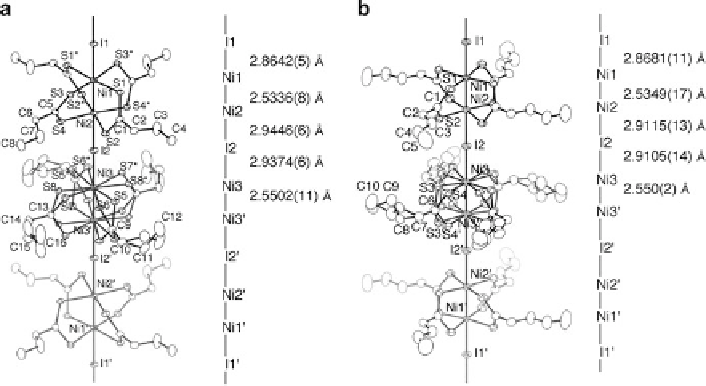
Fig. 9.32 1D chain structures of (a) [Ni
2
(
n
-PrCS
2
)
4
I]
1
(9) in the RT phase at 240 K and
(b) [Ni
2
(
n
-BuCS
2
)
4
I]
1
(10) at 290 K with an atomic numbering scheme and relevant interatomic
distances (thermal ellipsoid set at the 50 % probability level) [
38
]
a low-temperature (LT) phase [
80
]. In the compound 10, on the other hand, three
relatively sharp anomalies around 260 K were observed, while a broad anomaly was
observed around 135 K [
81
] These facts indicate the existence of three phases of
room temperature (RT), middle temperature (MT), and low temperature (LT). The
1D chain structures of 9 for the RT phase at 240 K and 10 at 290 K are shown in
Fig.
9.32
[
38
].
The RT phase of 9 at 240 K and 10 at 290 K crystallize in the monoclinic space
group
C
2/
m
and tetragonal space group
P
4/
mnc
, respectively. Since the crystal
structures of 9 and 10 are very similar, the RT phase of 9 is described here.
These structures are also analogous to those of the RT phases of the platinum
compounds 3-5. The unit cell dimension
b
along the 1D chain in 9 consists of three
-Ni-Ni-I- units. Crystallographic mirror planes perpendicular to the 1D chain exist on
the I1 atoms and the midpoint of Ni3 and Ni3
0
atoms. Therefore, the ligand moieties
including sulfur atoms of Ni3-Ni3
0
units are disordered on two positions and the
twisting directions of two NiS
4
planes of adjacent dinuclear Ni1-Ni2 units in the
1D chain are opposite to each other. Two nickel atoms are bridged by four
dithiobutanato ligands and the Ni-Ni distances are Ni1-Ni2
¼
2.5336 (8) and
2.5502 (11)
˚
,whichare0.23
˚
shorter than the distances between
the mean planes defined by the four sulfur atoms (2.763 (3) and 2.781 (3)
˚
),
respectively. The twist angle between two NiS
4
planes are 28.9 (1)
for a
Ni1-Ni2 unit and 27.4 (2)
for a Ni3-Ni3
0
unit, respectively. Three Ni-I
distances are Ni1-I1
Ni3-Ni3
0
¼
2.9374
(6)
˚
. Taking into account the differences in the Ni-I distances, the valence-
ordered state of the nickel atoms in the threefold periodic structure may be regarded
as -I
-Ni
3+
-Ni
2+
-I
-Ni
2.5+
-Ni
2.5+
-I
-Ni
2+
-Ni
3+
-I
-. In such a valence state, the
¼
2.8642(5), Ni2-I2
¼
2.9446(6), and Ni3-I2
¼
Search WWH ::

Custom Search