Chemistry Reference
In-Depth Information
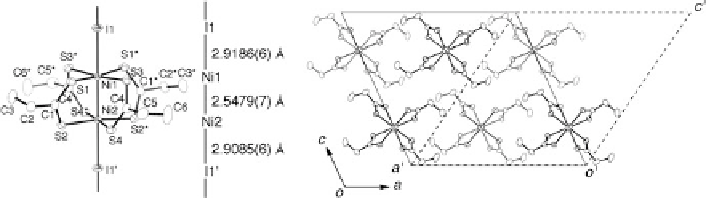
Fig. 9.31 (a) 1D chain structure of [Ni
2
(EtCS
2
)
4
I]
1
(8) at 292 K with an atomic numbering
scheme and relevant interatomic distances (thermal ellipsoid set at the 50 % probability level) [
38
].
(b) Packing diagrams projected down the
b
axis. A simple vectorial relationship between the unit
cell dimensions of [M
2
(EtCS
2
)
4
I]
1
(M
¼
Ni (8), Pt (2)) are found to be
a ¼a
0
,
b
0
b
,
c
0
¼ a þ c
, where the unit cell drawn with the dotted lines refers to the platinum compound 2
formation by the removal of an electron from the filled d
* orbital [
32
,
53
].
In contrast, the Ni-Ni distance appears to be strongly influenced by the interaction
with the surrounding atoms rather than by the formal oxidation number of the nickel
atoms, i.e., Ni-I interaction in 8, intermolecular Ni
s
S interaction in [Ni
2
(MeCS
2
)
4
]
[
37
], and intermolecular Ni
Ni interaction in [Ni
2
(EtCS
2
)
4
][
79
], in addition
to the packing effect including the twist angle of the two NiS
4
planes. Therefore,
the tendency for the M-M distance to shorten with increasing the formal
oxidation state is not a criterion for the dinickel complex. The two Ni-I distances
are Ni1-I1
2.9085(6)
˚
, and the bridging iodine atom
slightly deviates from the midpoint of the dinuclear units. Generally, the Ni
2+
-I
distance is longer than that of Ni
3+
-I
since a pair of electrons occupies thed
z
2
orbital
of Ni
2+
, and therefore the difference between the Ni-I distances indicates a charge
disproportionation of the nickel atoms. Taking into account the distinct difference
of Ni-I distances, the valence-ordered state of 8 at RT should be assigned to
a charge-polarization (CP) state of -Ni
(2.5
d
)+
-Ni
(2.5+
d
)+
-I
-Ni
(2.5
d
)+
-Ni
(2.5+
d
)+
-I
-(
2.9186(6) and Ni2-I1
0
¼
¼
d
0.5) close to an averaged-valence (AV) state.
In the case of
[Ni
2
(MeCS
2
)
4
I]
1
(7), the shorter interchain S
S distances are 3.619(8) and
3.810(5)
˚
, which are relatively close to the van der Waals contact distance
between sulfur atoms (3.60
˚
) and it can therefore be regarded as having a two-
dimensional interaction [
37
]. Whereas, the shorter interchain S
S distances in
8 are 4.359(1) and 4.973(1)
˚
, indicating no interchain S
S contacts. Therefore,
one-dimensionality of 8 is enhanced by the introduction of the ethyl group into
dithiocarboxylato ligand instead of the methyl group.
9.3.2.2
[Ni
2
(RCS
2
)
4
I]
1
RT Phases of [Ni
2
(RCS
2
)
4
I]
1
(R
¼ n
-Pr (9),
n
-Bu (10))
Ikeuchi and Saito et al. have reported the heat capacities of 9 and 10 measured by
adiabatic calorimetry [
80
,
81
]. For the compound 9, a first-order phase transition
observed at 205.6 K, indicating the existence of a room-temperature (RT) phase and
Search WWH ::

Custom Search