Chemistry Reference
In-Depth Information
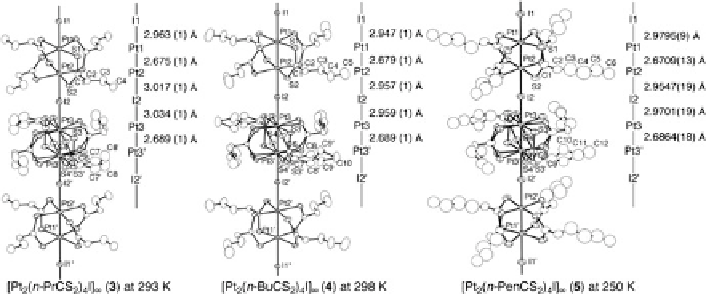
Fig. 9.5 1D chain structures of [Pt
2
(RCS
2
)
4
I]
1
(R
¼ n
-Pr (3),
n
-Bu (4),
n
-Pen (5)) in the RT
phase with an atomic numbering scheme and relevant interatomic distances (thermal ellipsoid set
at the 50 % probability level) [
33
-
35
]. Crystallographic mirror planes perpendicular to the 1D
chain exist on the I1 atoms and the midpoint of Pt3 and Pt3
0
atoms (i.e.,
z ¼
0, 0.5, 1)
mirror planes perpendicular to the 1D chain exist on the I1 atoms and the midpoint
of Pt3 and Pt3
0
atoms (i.e.,
z ¼
0, 0.5, 1). Therefore, the ligand moieties including
sulfur atoms bonded to Pt3-Pt3
0
units are disordered on two positions and the
twisting directions of two PtS
4
planes of adjacent dinuclear Pt1-Pt2 units in the
1D chain are opposite to each other. Two platinum atoms are bridged by four
dithiohexanato ligands in a paddle-wheel fashion with Pt-Pt distances of Pt1-Pt2
¼
2.689 (1)
˚
. The twist angles between two PtS
4
planes
are 21.45(7)
for a Pt1-Pt2 unit and
2.679 (1) and Pt3-Pt3
0
¼
20.3(2)
for a Pt3-Pt3
0
unit, respectively.
¼
2.959 (1)
˚
. Generally, a Pt
2+
-I
distance is greater than a Pt
3+
-I
distance
because the d
z
2
orbital of the Pt
2+
site is occupied by a pair of electrons. Therefore,
the difference between Pt-I bonds enables us to determine the valence state of Pt
atoms. Taking into account the small but significant differences in the Pt-I and
Pt-Pt distances, the valence-ordered state of the platinum atoms in the threefold
periodic structure may be regarded as an extreme model of -I
-Pt
2+
-Pt
3+
-I
-Pt
2.5+
-Pt
2.5+
-I
-Pt
3+
-Pt
2+
-I
-. In such a valence state, a band gap formation occurs
due to the structural distortion and the unpaired electrons on the adjacent Pt
3+
sites
are expected to take a singlet state due to the strong antiferromagnetic coupling
through the bridging iodine atom. However, as described later, the RT and HT
phases of 4 and 5 are a metallic or highly conductive paramagnetic state and diffuse
streaks with the twofold periodicity of a -Pt-Pt-I- period were observed in the
those RT and HT phases, indicating the presence of the valence fluctuation having
the twofold periodicity of a -Pt-Pt-I- period [
35
,
54
]. The RT phases of 4 and 5
should consequently be assigned to the valence-ordered state close to the AV state.
On the other hand, the compound 3 at room temperature exhibits the Bragg
reflections with the twofold periodicity of a -Pt-Pt-I- period instead of diffuse
streaks, indicating that 3 takes the valence-localized state corresponding to ACP or
¼
¼
The three Pt-I distances are Pt1-I1
2.947 (1), Pt2-I2
2.957 (1), and Pt3-I2
Search WWH ::

Custom Search