Chemistry Reference
In-Depth Information
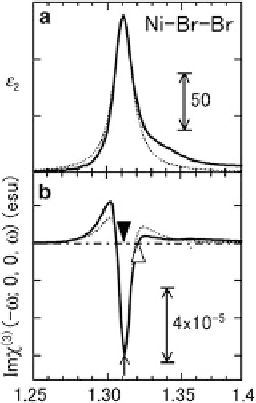
Fig. 7.4 Dielectric constants
e
2
(a) and its electric field-
induced change
De
2
(b).
Solid
line
is the experimental
results and the
dotted line
is
the fitting result using a
discrete-level model. Adapted
from [
10
]
most typical reference is a plate of SiO
2
. The details of the measurements
procedures of the
Z
-scan were reported in [
13
,
14
].
7.3 Third-Order Optical Nonlinearity in Ni-X Chain
Compounds
7.3.1 Electroreflectance Spectroscopy
Linear optical constants of Ni-X chain compounds were evaluated by the
measurements of polarized reflectivity (
R
) spectra. Figure
7.4a
shows the spectrum
of the imaginary part of the dielectric constants,
e
2
, in [Ni(chxn)
2
Br]Br
2
, which was
obtained from the
R
spectrum by the KK transformation. A sharp peak is observed
at around 1.3 eV, which is assigned to the CT transition from Br to Ni mentioned
above [
8
,
9
].
Electroreflectance (ER) spectra of three Ni-X chain compounds, [Ni(chxn)
2
Br]
Br
2
(abbreviated as Ni-Br-Br, hereafter), [Ni(chxn)
2
Cl]Cl
2
(Ni-Cl-Cl), and [Ni
(chxn)
2
Cl](NO
3
)
2
(Ni-Cl-NO
3
), were reported first by Kishida et al. [
3
]. The
electric field change
De
2
of
e
2
obtained from the ER spectrum was shown in
Fig.
7.4b
. Around the
e
2
are observed. The
positive and negative signals indicate the increase and the decrease of
e
2
peak, oscillation-type changes of
e
2
, respec-
w
ð
3
Þ
as mentioned in Sect.
7.3.1
,
which is scaled by the right axis in Fig.
7.4b
. The max
tively.
De
2
is converted to the imaginary part of
w
ð
3
Þ
ðo;
j
Im
0
;
0
; oÞj
10
5
esu in Ni-Br-Br. The
ER spectroscopy was performed in other Ni-X compounds. The max
w
ð
3
Þ
j
(hereafter, abbreviated as max
j
Im
) reaches 9
w
ð
3
Þ
j
values of Ni-X compounds are plotted in Fig.
7.5
[
3
,
10
], together with those of
j
Im
Search WWH ::

Custom Search