Biomedical Engineering Reference
In-Depth Information
absent in the case of synthetic hydroxyapatite. Similarly, two well-defined peaks
present at 931 cm
−1
for calcium hydroxide and 1079 cm
−l
(O-H symmetric) were
completely absent in the spectrum of synthetic hydroxyapatite [24].
Comparison of Natural and Synthetic Apatites
Comparing the spectra of natural bones with that of synthetic hydroxy-
apatite, it is apparent that there are significant structural differences. The
spectra of bone tissue provide a baseline from which to improve the existing
synthetic hydroxyl/carbonate/substituted apatite materials.
The spectrum of calcium carbonate (Figures 7.4 and 7.5 and Table 7.1)
showed that the peaks at 1433, 1062, and 710 cm
−1
(carbonate) were also pres-
ent in the spectrum of bone, indicating that the inorganic phase of the bone
contains a substantial amount of carbonate. In contrast, commercial synthetic
hydroxyapatite material has only a trace of carbonate moiety.
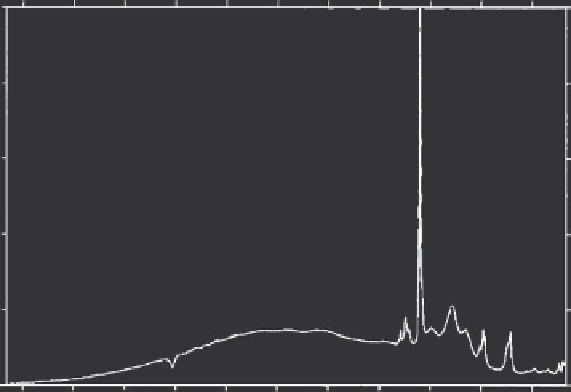
Calcined bone has also been considered in the past as an implantable and
has been of interest to the spectroscopic community. The spectrum of cal-
cined bone is given in Figure 7.6, where the absence of a carbonate group is
evident. The carbonate group is less stable compared to the phosphate group
and is destroyed during the calcination process.
1.01
0.81
0.61
0.41
0.21
0.01
3300
3000
2700
2400
2100 1800
Raman Shift (cm
-1
)
1500
1200
900
600
300
Figure 7.6
Raman spectra of calcined bone.

Search WWH ::

Custom Search