Chemistry Reference
In-Depth Information
hydrogen-bonding O
2
and O
6
atoms on outside surface of the helix with only the ring
oxygen pointing inwards. Hydrogen bonding between aligned chains causes retro-
gradation and releases some of the bound water (syneresis). The aligned chains may
then form double stranded crystallites that are resistant to amylases. These possess
extensive inter- and intra-strand hydrogen bonding, resulting in a fairly hydropho-
bic structure of low solubility. Single helix amylose behaves similar to the cyclodex-
trins, by possessing a relatively hydrophobic inner surface that holds a spiral of water
molecules, which are relatively easily to be replaced by hydrophobic lipid or aroma
molecules. It is also responsible for the characteristic binding of amylose to chains of
charged iodine molecules.
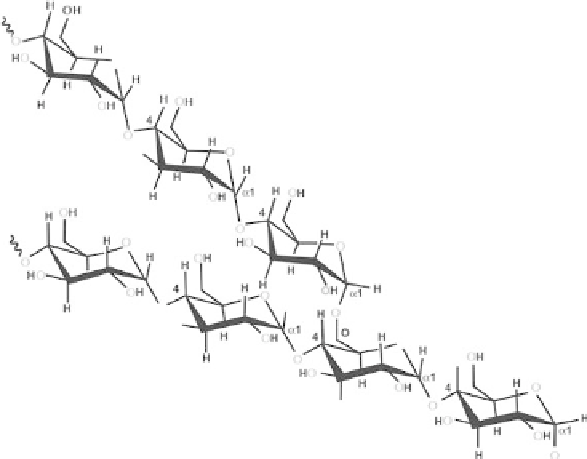
Amylopectin
Amylopectin is formed by non-random α-(1, 6) branching of the amylose-type α-(1,
4)-D-glucose structure (Scheme 3.2). Each amylopectin molecule contains a million
or so residues, about 5% of which form the branch points. There are usually slightly
more outer unbranched chains (called A-chains) than inner branched chains (called B
chains). There is only one chain (called the C-chain) containing the single reducing
group. The-chains generally consist of residues between 13 and 23. There are two
main fractions of long and short internal B-chains with the longer chains (greater than
about 23-35 residues) connecting between clusters and the shorter chains similar in
length to the terminal A-chains. Each amylopectin molecule contains up to two million
glucose residues in a compact structure with hydrodynamic radius of 21-75 nm. The
molecules are oriented radially in the starch granule and as the radius increases so does
the number of branches required in filling up the space, and the consequent formation
of concentric regions of alternating amorphous and crystalline structures.
Scheme 3.2.
Structure of amylopectin.
















Search WWH ::

Custom Search