Biomedical Engineering Reference
In-Depth Information
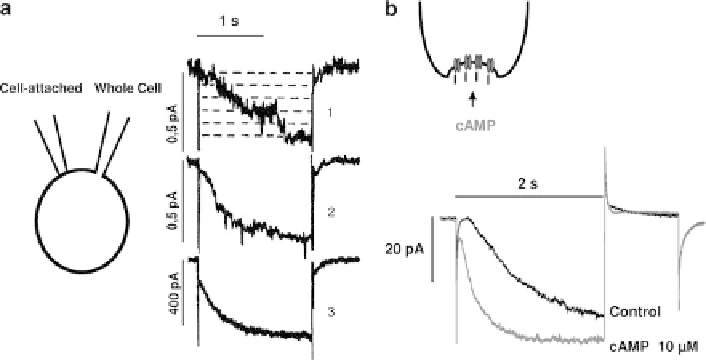
Fig. 2 (a) Representative recordings of pacemaker currents from whole-cell and cell-attached
configurations.
1
- Single channel traces recorded from a cell-attached patch during hyperpolari-
zation to
32 mV.
2
- Average of nine cell-attached traces.
3
- Whole-cell pacemaker current recorded with a second pipette during the same pulses (modified
from [
29
]). (b) Action of cAMP on
I
f
activation in an inside-out macropatch. The
I
f
current was
activated on hyperpolarization to
102 mV from a holding potential of
95 mV in a macropatch exposed to cAMP (10
M) on the
m
inside as represented in the
inset
conductances reported for HCN isoforms are also very elevated (13- to 35-fold),
and while the reason for this major discrepancy was not identified, it could be
explained by the different cell preparations or experimental conditions (i.e., patch
configurations) used. However, ensemble records shown by Michels and
collaborators [
33
] for HCN isoforms and native h-current seem flat and do not divulge
any time dependence, reflecting an instantaneous rather than a time-dependent
behavior. Thus, it remains unclear whether pacemaker channels could exhibit two
distinct conductances and/or different kinetics.
4 Role
As mentioned above, HCN channels generate and/or regulate neuronal and cardiac
excitability. Several physiological roles have been ascribed to HCN channels,
which are the consequences of their particular biophysical properties (see [
16
] for
review).
In general, HCN channels engender and regulate neuronal and cardiac firing
rates. Besides acting as a pacemaker, the HCN current also functions as a regulator
of resting potential and membrane resistance. The current stabilizes the resting
membrane potential because small hyperpolarizations activate the pacemaker
channels, whose inward currents depolarize the cell. This depolarization, as a