Biomedical Engineering Reference
In-Depth Information
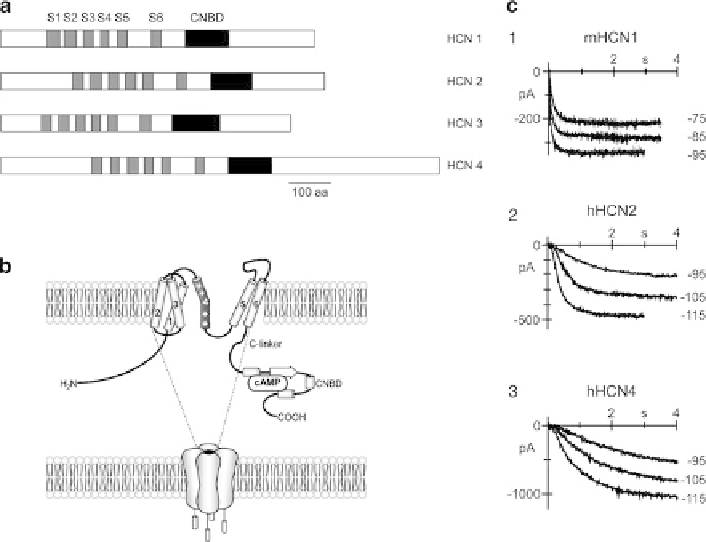
Fig. 1 (a) The four isoforms of the HCN family. The six transmembrane segments S1-S6 are
numbered 1-6, CNBD indicates the cyclic nucleotide-binding domain. (b) HCN channels are
tetramers. One monomer is composed of six transmembrane segments including the voltage sensor
(S4) and the pore region between S5 and S6. The pore region contains the selectivity filter carrying
the GYG motif. The COOH terminal channel domain is composed of the C-linker and the cyclic
nucleotide-binding domain (CNBD). (c) Kinetic properties of the different HCN isoforms. Acti-
vation traces recorded on hyperpolarization to the indicated voltages of HEK-293 cells expressing
mouse HCN1 (1), human HCN2 (2), and human HCN4 (3) channels (modified from [
13
])
voltage-dependent machinery, HCN proteins are characterized by a C-terminus
domain, which contains consensus sequences able to bind with cyclic nucleotides
(a 120 amino acid-long cyclic nucleotide binding domain, CNBD). From a func-
tional point of view, as detailed below, channel activation is triggered by membrane
hyperpolarization. The latter is facilitated by the prior binding of a cyclic nucleotide
to the CNBD region. A high degree of conservation between the four HCN isoforms
is observed in the transmembrane region, in the C-terminus CNBD, and in the
peptide located between the sixth transmembrane domain and the CNBD (an 80
amino acid-long C-linker). In contrast, a high diversity is found in the N-terminal
region and the C-terminal region downstream of the CNBD.
HCN subunits must assemble as tetramers, organized around the central pore
region, to constitute functional channels. Biophysical and pharmacological
properties depend on subunit composition and heterotetramerization may be
necessary to constitute channels with current properties identical to native currents
[
17
,
18
].